Peptide Imidazolones
Reflecting work in the VanVeller Lab
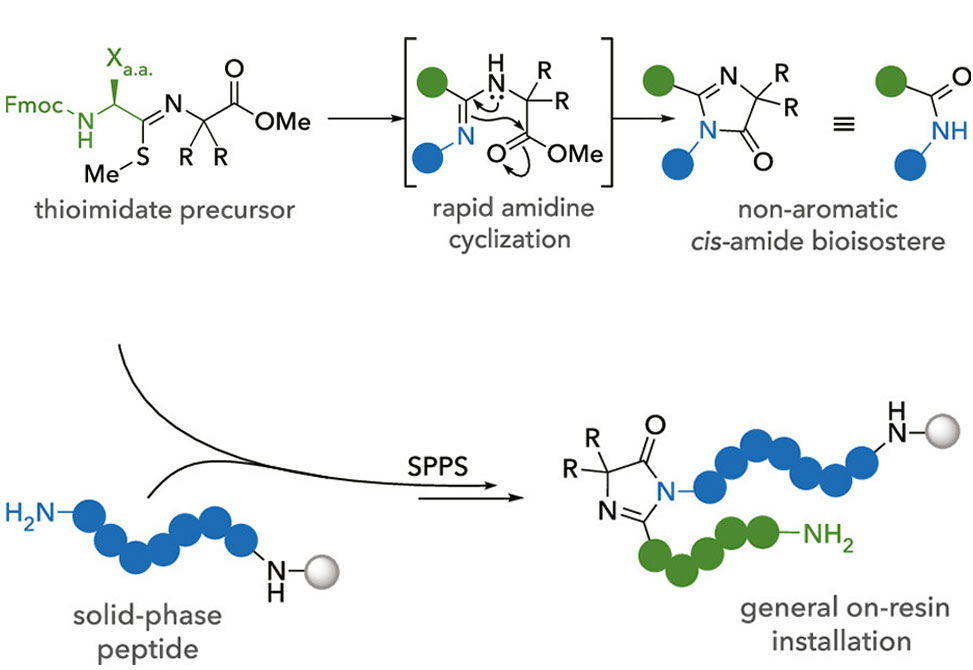
Imidazolones represent an important class of heterocycles present in a wide range of pharmaceuticals, metabolites, and bioactive natural products and serve as the active chromophore in green fluorescent protein. Recently, imidazolones have received attention for their ability to act as a nonaromatic amide bond bioisotere which improves pharmacological properties.

The article authors, Brendan J. Wall, Krishna K. Sharma, Emily A. O’Brien, Aaron Donovan, and Brett VanVeller
Published in JACS, researchers in the VanVeller Group at Iowa State University, present a tandem amidine installation and cyclization with an adjacent ester to yield (4H)-imidazolone products. Using amino acid building blocks, we can access the first examples of α-chiral imidazolones that have been previously inaccessible. Additionally, the group's method is amenable to on-resin installation which can be seamlessly integrated into existing solid-phase peptide synthesis protocols. Finally, this shows that peptide imidazolones are potent cis-amide bond surrogates that preorganize linear peptides for head-to-tail macrocyclization.
This work represents the first general approach to the backbone and side-chain insertion of imidazolone bioisosteres at various positions in linear and cyclic peptides.