Peptide Permeation
Reflecting work in the Reflecting recent work in the Su, Partridge and Verma Labs
Macrocyclic peptides show promise in targeting high-value therapeutically relevant binding sites due to their high affinity and specificity. However, their clinical application is often hindered by low membrane permeability, which limits their effectiveness against intracellular targets. Previous studies focused on peptide conformations in various solvents, leaving a gap in understanding their interactions with and translocation through lipid bilayers.
Addressing this, a study published in JACS, performed by researchers from Merck, Quantitative Biosciences, and the Bioinformatics Institute (Agency for Science Technology and Research, A*STAR, Singapore), explores the membrane interactions of stapled peptides, a subclass of macrocyclic peptides, using solid-state nuclear magnetic resonance, ssNMR, spectroscopy and molecular dynamics, MD, simulations.
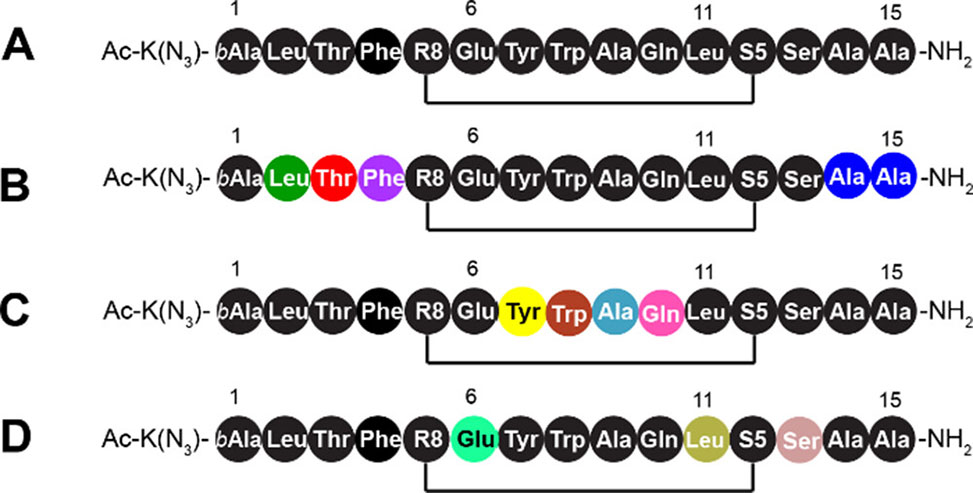
Figure 1. Sequences and isotopic labeling of the stapled peptides employed here. (A) The natural abundance version of ATSP-7041M, a staple peptide named P53 for convenience. (B, C) ATSP-7041M with uniformly isotopically labeled 13C, 15N-labeled residues. These include P53 peptides isotopically labeled at Leu2, Thr3, Phe4, Ala14, and Ala15, named LTFAA-P53 (B); Tyr7, Trp8, Ala9, and Gln10, named YWAQ-P53 (C); and Glu6, Leu11, and Ser14, named ELS-P53 (D).
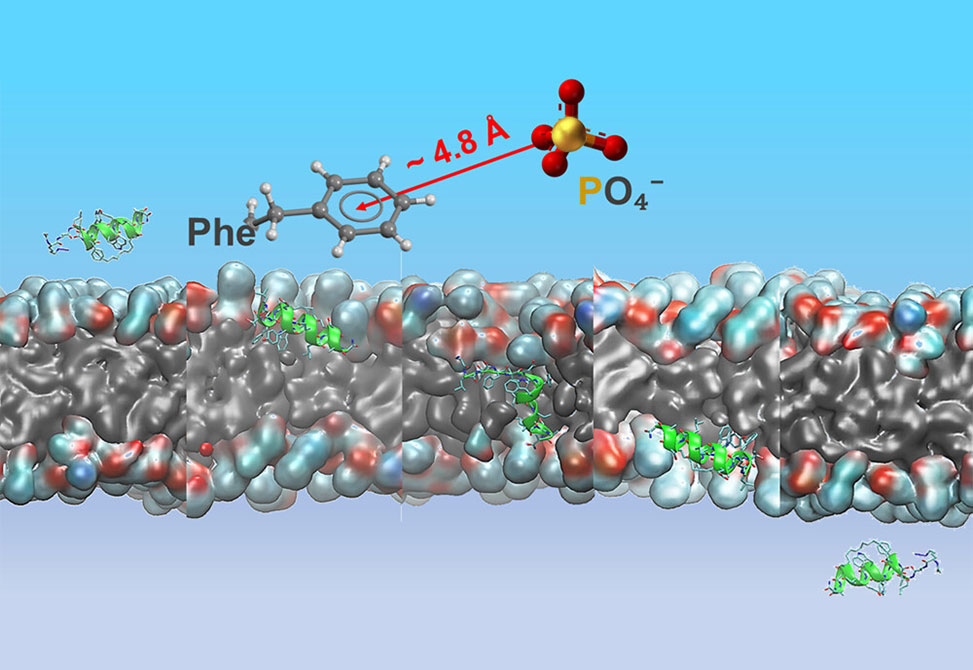
The group members conducted ssNMR measurements on ATSP-7041M, a prototypical stapled peptide, to understand its interaction with lipid membranes, leading to an MD-informed model for peptide membrane permeation. Their findings reveal that ATSP-7041M adopts a stable α-helical structure upon membrane binding, facilitated by a cation−π interaction between its phenylalanine side chain and the lipid headgroup. This interaction makes the membrane-bound state energetically favorable, facilitating membrane affinity and insertion.
The bound peptide displayed asymmetric insertion depths, with the C-terminus penetrating deeper, approximately 9 Å, than the N-terminus, approximately 4.3 Å, relative to the lipid headgroups. Contrary to expectations, peptide dynamics was not hindered by membrane binding and exhibited rapid motions similar to cell-penetrating peptides. These dynamic interactions and peptide–lipid affinity appear to be crucial for membrane permeation. MD simulations indicated a thermodynamically stable transmembrane conformation of ATSP-7041M, reducing the energy barrier for translocation.
This study offers an in silico view of ATSP-7041M’s translocation from the extracellular to the intracellular region, highlighting the significance of peptide–lipid interactions and dynamics in enabling peptide transit through membranes.