Peptide Receptors
Reflecting work in the Checco Lab
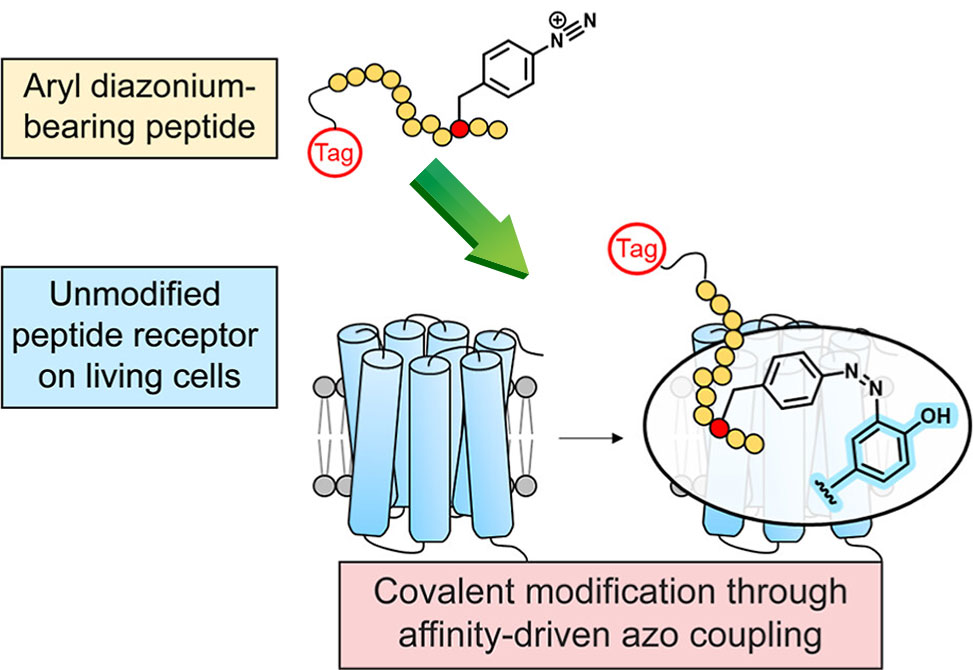
Peptide–receptor interactions play critical roles in a wide variety of physiological processes. Methods to link bioactive peptides covalently to unmodified receptors on the surfaces of living cells are valuable for studying receptor signaling, dynamics, and trafficking and for identifying novel peptide–receptor interactions.
Sheryl Sharma, Michael J. Naldrett, Makayla J. Gill, and James W. Checco from the University of Nebraska-Lincoln, published in JACS, utilize peptide analogues bearing deactivated aryl diazonium groups for the affinity-driven labeling of unmodified receptors. They demonstrate that aryl diazonium-bearing peptide analogues can covalently label receptors on the surface of living cells using both the neurotensin and the glucagon-like peptide 1 receptor systems. Receptor labeling occurs in the complex environment of the cell surface in a sequence-specific manner.

The Checco Group
The researchers further demonstrate the utility of this covalent labeling approach for the visualization of peptide receptors by confocal fluorescence microscopy and for the enrichment and identification of labeled receptors by mass spectrometry-based proteomics. Aryl diazonium-based affinity-driven receptor labeling is attractive due to the high abundance of tyrosine and histidine residues susceptible to azo coupling in the peptide binding sites of receptors, the ease of incorporation of aryl diazonium groups into peptides, and the relatively small size of the aryl diazonium group.
This approach should prove to be a powerful and relatively general method to study peptide–receptor interactions in cellular contexts.