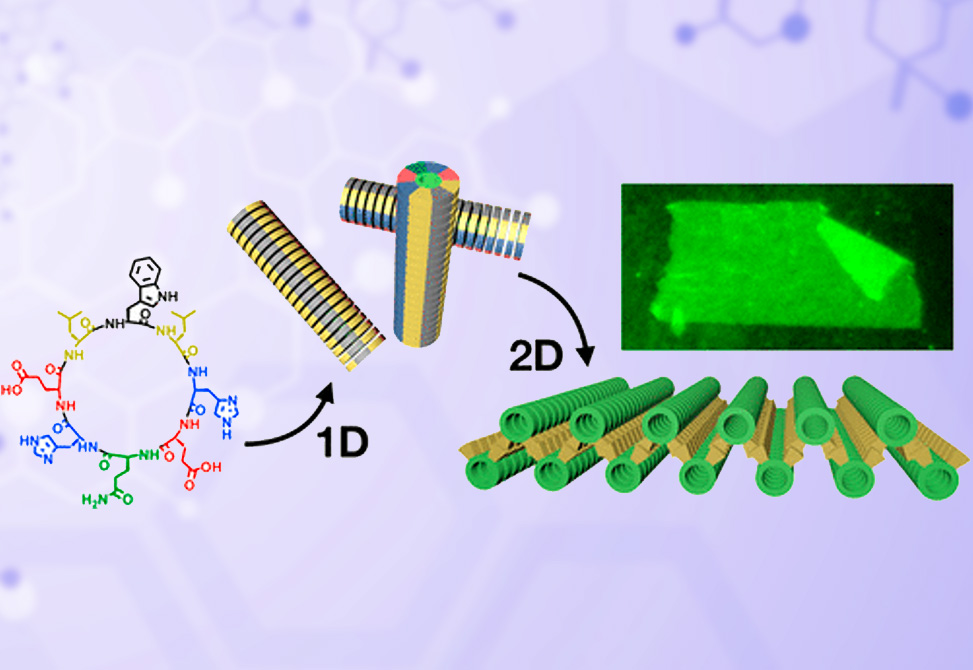
Peptide Self Assembly
Reflecting recent work in the
Despite recent developments in two-dimensional self-assembly, most supramolecular 2D materials are assembled by tedious methodologies, with complex surface chemistry and small sizes.
In this report Insua and Montenegro show that D/L-alternating cyclic peptides undergo one-dimensional self-assembly into amphiphilic nanotubes, which subsequently arrange as tubular bilayers to form giant nanosheets in the mesoscale.
Reversible transitions between the assembled, dispersed, and aggregated states of these nanosheets can be triggered by external stimuli. The characteristic flexibility, defined chemical topology, and length scale of these nanosheets set a clear distinction between this new supramolecular architecture and previously reported 2D nanostructures.
The sequential 1D-to-2D self-assembly of peptides described here provides a conceptually new approach to achieve two-dimensional materials with hierarchical organization. These giant nanosheets represent one of the largest 2D supramolecular materials ever made, with potential application as long-range molecular transporters, responsive surfaces, and (bio)sensors.