Peptide Synthesis
Reflecting work in the
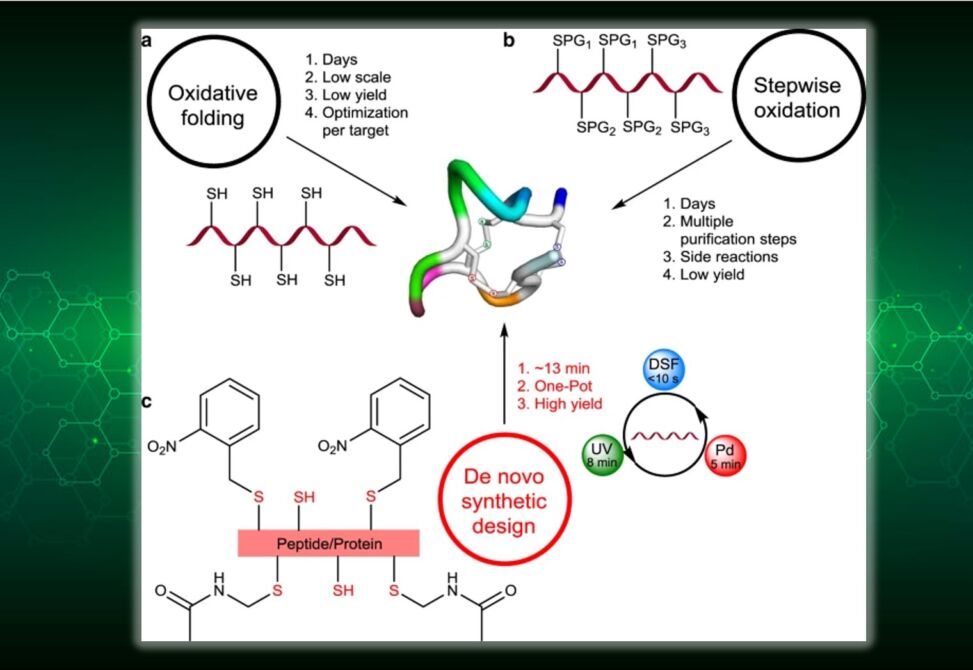
Despite six decades of efforts to synthesize peptides and proteins bearing multiple disulfide bonds, this synthetic challenge remains an unsolved problem in most targets (e.g., knotted mini proteins).
Here the authors show a de novo general synthetic strategy for the ultrafast, high-yielding formation of two and three disulfide bonds in peptides and proteins. They developed an approach based on the combination of a small molecule, ultraviolet-light, and palladium for chemo- and regio-selective activation of cysteine, which enables the one-pot formation of multiple disulfide bonds in various peptides and proteins.
They prepared bioactive targets of high therapeutic potential, including conotoxin, RANTES, EETI-II, and plectasin peptides and the linaclotide drug. They anticipate that this strategy will be a game-changer in preparing millions of inaccessible targets for drug discovery.