Photoregulated Catalysis
Reflecting work in the Bandyopadhyay Lab
Precise external regulation of enzymatic activity remains a central goal in chemical biology, with wide-ranging implications for biotechnology, diagnostics, and medicine. Natural proteases govern countless biological processes, yet their lack of spatiotemporal control limits their direct application in engineered systems. Conventional strategies for modulating proteolysis, such as pH, temperature, or chemical effectors, offer little precision, and systemic protease inhibition carries a high risk of off-target toxicity. Light, by contrast, provides non-invasive, reversible, and highly localized control, making it an ideal external trigger for catalytic regulation.
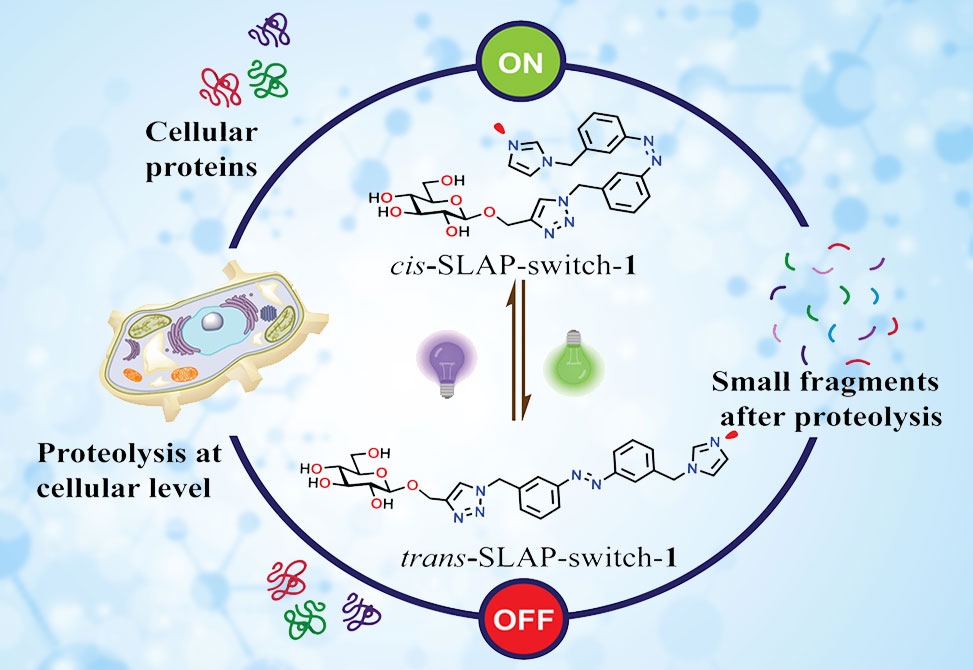
Building upon this principle, researchers from the Bandyopadhyay Group at the Department of Chemical Sciences, Indian Institute of Science Education and Research, IISER, in Kolkata, India, published in Angewandte Chemie International Edition, have developed a small-molecule light-activated peptidase, SLAP, that functions as an enzyme mimic. The design integrates three critical components: 1| a glucose unit to provide hydrogen-bonding and transition-state stabilization, 2| an imidazole residue to serve as a general base, and 3| an azobenzene photoswitch that dynamically modulates the spatial relationship between these catalytic elements. Upon photoisomerization, the system undergoes a dramatic change in activity, enabling light-gated proteolysis of small molecules, peptides, and cellular proteins.
The SLAP catalyst is inspired by natural proteases like papain and trypsin, wherein catalysis relies on the proximity between a nucleophilic residue, serine or cysteine, and a general base, histidine. In SLAP-switch-1, the primary hydroxyl group of glucose substitutes as the nucleophile, while the imidazole moiety provides base activation. These two components are tethered by a bifunctional azobenzene linker, whose trans state holds them ~14 Å apart, inactive, and whose cis state contracts this distance to ~1.8 Å, active.This structural rearrangement establishes short hydrogen bonds, <2.5 Å, essential for catalytic efficiency. Beyond mere positioning, the glucose unit also offers a substrate-binding handle through multiple hydrogen bonds and contributes to transition-state stabilization. Such fine molecular engineering echoes Feringa’s vision of artificial molecular machines, where motion and catalysis are coupled through photonic energy inputs.
Spectroscopic studies confirmed robust and reversible photoswitching. The trans isomer exhibited characteristic π–π*, 319 nm, and n–π*, 430 nm, absorption bands, which shifted upon UV, 366 nm, irradiation to yield a cis-enriched photostationary state, ~62% cis. Blue light, 466 nm, reversed the process, regenerating the trans state, >90%.
Importantly, SLAP-switch-1 displayed fatigue resistance over multiple irradiation cycles, with no significant degradation across ten switching events. Thermodynamic analysis revealed that the cis isomer possesses an unusually long half-life, ~74 days at room temperature, attributed to stabilizing intramolecular hydrogen bonds. This ensures both durability and practicality for repeated light-controlled catalysis.
The catalytic capacity of SLAP-switch-1 was first evaluated with p-nitroacetanilide, PNA, as a model substrate. In the presence of the cis isomer, UV–vis monitoring revealed rapid disappearance of the 315 nm absorption band, substrate, and emergence of a 385 nm band, product, p-nitroaniline. The cis isomer achieved a ~300-fold enhancement in hydrolytic rate relative to the trans state or catalyst-free controls, underscoring the profound influence of spatial proximity on activity.
Subsequent assays with a FRET-based peptide substrate bearing a tryptophan as a fluorophore and a DNP unit as a quencher, DNP-PLGMWSR, provided further evidence for the efficacy of the SLAP switch as a peptidase. Michaelis–Menten analysis yielded kcat = 1.94 min-1, KM = 0.12 mM, and a catalytic proficiency, kcat/kuncat, of ~103, values notable for a small-molecule mimic. LC–MS analysis confirmed the release of amino acid fragments, validating true peptide bond hydrolysis. Crucially, catalytic activity was shown to be reversibly switchable: alternating cycles of UV and visible light toggled the system between active and inactive states, enabling repeated on–off regulation of proteolysis.
To prove biological relevance, SLAP-switch-1 was introduced into HEK-293 cells, focusing on microtubule integrity as a readout. Confocal microscopy revealed significant loss of microtubule staining in cis-treated samples, while trans-treated and control cells retained intact cytoskeletons. Western blotting corroborated these findings, showing marked reduction of tubulin protein levels only under cis activation.
These results demonstrate that the catalyst functions not only in vitro but also within cellular contexts, achieving light-regulated proteolysis of structural proteins under physiological conditions. While the system is not inherently selective for tubulin, its proof-of-concept activity against a well-defined cytoskeletal target highlights the feasibility of extending small-molecule enzyme mimics into living systems.
The development of SLAP-switch-1 marks a significant advance in the field of artificial enzyme mimics. Unlike earlier catalytic antibodies or cyclodextrin-based systems, this design achieves high catalytic efficiency, broad substrate scope, and reversible regulation through a simple photoswitchable triad.
The implications span multiple domains. In basic science, light-gated peptidase mimics provide new tools for dissecting protein function with temporal and spatial precision. In biomedicine, they suggest future opportunities in targeted protein degradation, particularly in contexts such as tumor microenvironments or neurodegenerative protein aggregation, where conventional protease activity is either insufficient or deleterious. Finally, in bioengineering, such systems may serve as modular components for synthetic biology, drug delivery, or programmable biomaterials.
Challenges remain, notably the lack of intrinsic substrate specificity and the reliance on UV light, which can damage cells. Future efforts will focus on engineering variants responsive to near-infrared irradiation, compatible with deep-tissue applications, and on refining scaffold design to enhance selectivity toward disease-relevant targets. By uniting the principles of photochromism, proximity control, and transition-state stabilization, SLAP-switch-1 achieves light-controlled peptidase activity with remarkable efficiency. This artificial enzyme mimic represents a step toward programmable proteolysis, demonstrating the power of molecular design to replicate, and in key respects, surpass the regulatory sophistication of natural enzymes.