The Burkholderia cepacia complex, BCC, is a group of Gram-negative bacteria with a dual identity—recognized both for their opportunistic infections in cystic fibrosis, CF, patients and for their remarkable biosynthetic capabilities in producing bioactive secondary metabolites. Among these metabolites is AFC-BC11, an unusual lipopeptide with potent antifungal activity against major phytopathogens. Despite previous recognition of its biological relevance, both the chemical structure and biosynthetic logic of AFC-BC11 have remained unresolved — until now.
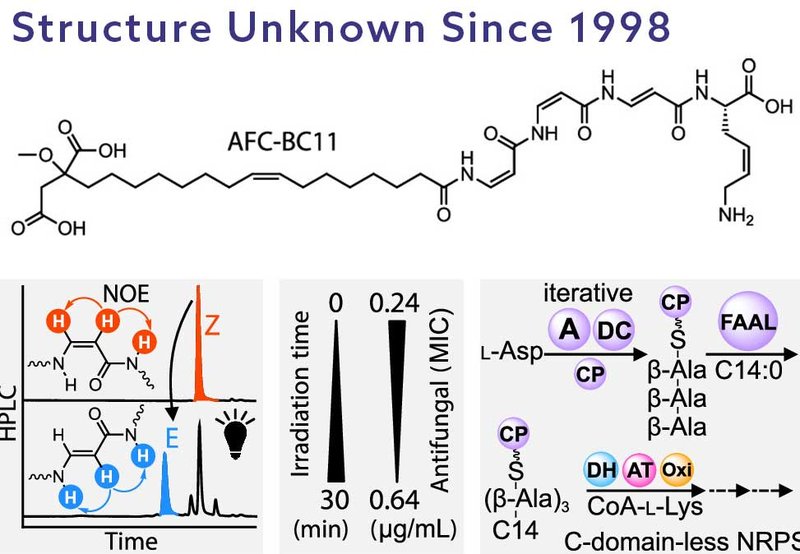
In this study, researchers from the Süssmuth group at the Technische Universität Berlin, published in the Journal of the American Chemical Society, elucidated the structure of AFC-BC11 as an N-acyl tetrapeptide with a distinctive architecture. The molecule is composed of a myristoylated, O-methylated malic acid, MMFA, moiety at the N-terminus, followed by a highly unusual trimer of dehydro-β-alanine, DBA, residues, and capped at the C-terminus with a γ,δ-dehydro-lysine, DHLys. This precise structural arrangement is critical for the molecule’s antifungal activity and is highly sensitive to light, with photoisomerization rapidly diminishing its bioactivity.
The antifungal potency of AFC-BC11 is notable, displaying minimal inhibitory concentrations, MICs, in the sub-microgram range, 0.24–0.72 μg/mL, against plant pathogenic fungi such as Colletotrichum kahawae and Pyrenophora teres f. teres. Its performance exceeds that of commercial fungicides like azoxystrobin. Intriguingly, the compound demonstrates no inhibitory activity against common human fungal pathogens or bacteria, highlighting a selective mode of action that may reflect ecological specialization toward plant-associated fungi.
Beyond its potent bioactivity, the biosynthesis of AFC-BC11 is remarkable for its departure from classical nonribosomal peptide synthesis, NRPS. Unlike canonical NRPS systems, which depend on modular enzymes featuring condensation, C, domains for peptide bond formation, the biosynthetic gene cluster, afc BGC, for AFC-BC11 is conspicuously devoid of these features. Instead, the assembly proceeds via a thiotemplated mechanism centered around a single carrier protein, AfcK, which mediates the sequential transfer of intermediates through an intricate network of autonomous enzymes.
The assembly process begins with AfcQ, an adenylation domain that iteratively activates L-aspartate, which is subsequently decarboxylated to β-alanine by AfcP. This iterative process constructs the unusual DBA trimer that forms the molecular core. The growing peptide chain is then N-acylated by a fatty acyl-AMP ligase, AfcA, which specifically recognizes a mono-carboxylic acid. The precise selection of a trimeric β-alanine scaffold for acylation demonstrates a highly controlled biosynthetic checkpoint that prevents chain extension beyond three residues.
Subsequent steps involve the transfer of the peptidyl intermediate to AfcL, a ketoacyl synthase III, KAS III-like enzyme that facilitates the coupling of the peptide chain to a L-lysine-CoA conjugate, an unusual mechanism distinct from classical peptide release strategies. The lysine residue is further oxidatively tailored by AfcC, introducing a critical double bond into the C-terminal DHLys moiety. Parallel tailoring enzymes, including AfcS, AfcF, and AfcT, orchestrate the maturation of the MMFA moiety through citrate incorporation, oxidation, and methylation.
Despite the successful reconstitution of the peptide core assembly, certain aspects of the MMFA tail biosynthesis remain enigmatic, particularly the precise timing and enzymology of the citrate-derived modifications. Gene knockout studies underscore the essentiality of multiple tailoring enzymes and membrane-associated proteins in the final assembly and export of AFC-BC11.
The discovery of AFC-BC11’s biosynthetic mechanism offers not only a new antifungal scaffold for agricultural applications but also provides profound insights into noncanonical peptide assembly pathways in bacteria. The reliance on iterative adenylation, thiotemplated chain elongation, and KAS III-mediated peptide ligation represents a rare and elegant deviation from the NRPS paradigm. Moreover, the conservation of the afc gene cluster across diverse Burkholderia species hints at possible roles beyond antifungal activity, potentially contributing to bacterial virulence in CF patients or offering adaptive advantages in soil and plant-associated niches.
This work establishes AFC-BC11 as a member of a new class of peptidomimetic antifungals and opens avenues for bioengineering efforts to optimize its activity, stability, and spectrum. Its exquisite light sensitivity—while a hurdle for laboratory handling—raises intriguing questions about whether this property plays a functional role in the ecological interactions of Burkholderia with its environment.