Proline Conformation
Reflecting work in the Zondlo Lab
Despite the importance of proline conformational equilibria, trans versus cis amide and exo versus endo ring pucker, on protein structure and function, there is a lack of convenient ways to probe proline conformation. 4,4-Difluoroproline, Dfp, was identified to be a sensitive 19F NMR-based probe of proline conformational biases and cis–trans isomerism. Within model compounds and disordered peptides, the diastereotopic fluorines of Dfp exhibit similar chemical shifts, ΔδFF = 0–3 ppm, when a trans X–Dfp amide bond is present. In contrast, the diastereotopic fluorines exhibit a large, ΔδFF = 5–12 ppm, difference in chemical shift in a cis X–Dfp prolyl amide bond.
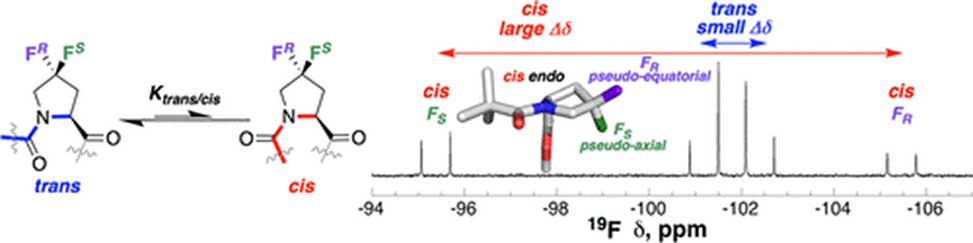
DFT calculations, X-ray crystallography, and solid-state NMR spectroscopy indicated that ΔδFF directly reports on the relative preference of one proline ring pucker over the other: a fluorine which is pseudo-axial, in essence, the pro-4R-F in an exo ring pucker, or the pro-4S-F in an endo ring pucker, is downfield, while a fluorine which is pseudo-equatorial, in essence, pro-4S-F when exo, or pro-4R-F when endo, is upfield.
Thus, when a proline is disordered, a mixture of exo and endo ring puckers, as at trans-Pro in peptides in water, it exhibits a small Δδ. In contrast, when the Pro is ordered, such as, when one ring pucker is strongly preferred, as in cis-Pro amide bonds, where the endo ring pucker is strongly favored, a large Δδ is observed. Dfp can be used to identify inherent induced order in peptides and to quantify proline cis–trans isomerism.

The Zondlo Group
Using Dfp, researchers in the Zondlo Lab at the University of Delaware, published in Biochemistry, discovered that the stable polyproline II helix, PPII, formed in the denatured state, 8 M urea, exhibits essentially equal populations of the exo and endo proline ring puckers. In addition, the data with Dfp suggested the specific stabilization of PPII by water over other polar solvents.
These data strongly support the importance of carbonyl solvation and n → π* interactions for the stabilization of PPII. Dfp was also employed to quantify proline cis–trans isomerism as a function of phosphorylation and the R406W mutation in peptides derived from the intrinsically disordered protein tau. Dfp is minimally sterically disruptive and can be incorporated in expressed proteins, suggesting its broad application in understanding proline cis–trans isomerization, protein folding, and local order in intrinsically disordered proteins.
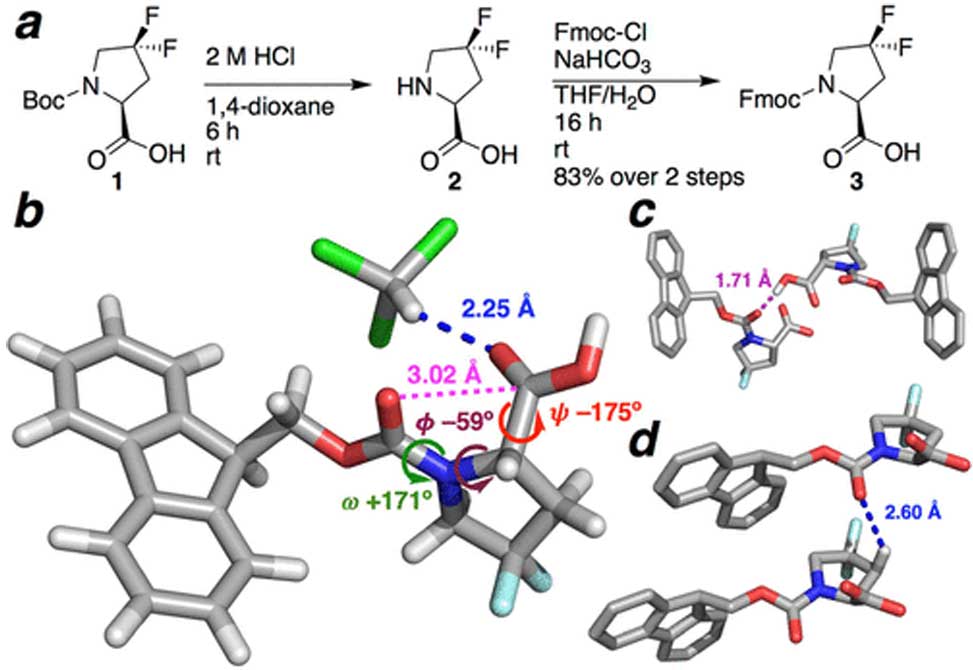 a Synthesis of Fmoc-Dfp–OH (3) from commercially available Boc-Dfp–OH (1). b Crystal structure (100 K data collection) of Fmoc-Dfp–OH·CHCl3. The structure exhibited a close C–H/O interaction (blue) between the carboxylic acid carbonyl and the C–H of chloroform. c Crystal assembly of Fmoc-Dfp-OH is mediated in part via hydrogen bonding between the carboxylic acid hydrogen and the carbamate carbonyl of an adjacent molecule. d Intermolecular C–H/O interaction between Pro C–Hβ and an adjacent carbonyl. Bond lengths to hydrogen were normalized in Mercury.
a Synthesis of Fmoc-Dfp–OH (3) from commercially available Boc-Dfp–OH (1). b Crystal structure (100 K data collection) of Fmoc-Dfp–OH·CHCl3. The structure exhibited a close C–H/O interaction (blue) between the carboxylic acid carbonyl and the C–H of chloroform. c Crystal assembly of Fmoc-Dfp-OH is mediated in part via hydrogen bonding between the carboxylic acid hydrogen and the carbamate carbonyl of an adjacent molecule. d Intermolecular C–H/O interaction between Pro C–Hβ and an adjacent carbonyl. Bond lengths to hydrogen were normalized in Mercury.

