Quorum Sensing Redux
Reflecting work in the The Tal-Gan Group
Streptococcus constellatus, a member of the Streptococcus anginosus group, SAG, is an opportunistic pathogen commonly found in the human oral and gastrointestinal microbiota. While often commensal, it has increasingly been implicated in severe purulent infections, particularly in immunocompromised individuals. Despite its emerging clinical relevance, the molecular pathways regulating its behavior remain poorly characterized. In this comprehensive study, published in Biochemistry as an ASAP article, the Tal-Gan group at the University of Nevada at Reno, elucidates the quorum sensing, QS, mechanisms that govern genetic competence in S. constellatus, offering unprecedented insight into the structure-function relationships of its competence-stimulating peptide, CSP, and downstream regulatory effects.
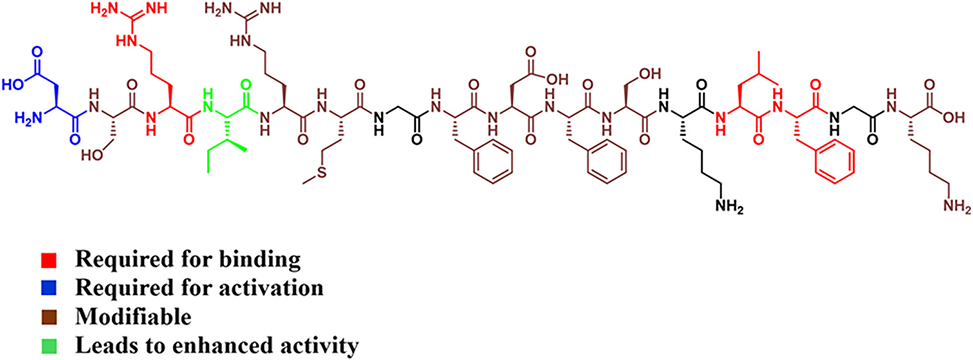
The team confirms that S. constellatus encodes a canonical comABCDE regulon—a QS circuit common in streptococci—which regulates the development of competence via CSP-mediated signaling. The mature CSP was identified as a 16-residue peptide, DSRIRMGFDFSKLFGK, through genomic analysis, chemical synthesis, and direct isolation from cell-free supernatants using RP-HPLC and mass spectrometry. Synthetic and isolated forms of the peptide were shown to be chemically identical and functionally potent in activating QS through the ComD histidine kinase receptor.
To quantitatively assess QS activity, the researchers in the Tal-Gan team developed a luciferase-based QS reporter strain and a ΔcomC mutant in which endogenous CSP production is eliminated. These tools enabled precise dissection of CSP analog activity without background interference. A full alanine scan and d-amino acid scan of the native CSP sequence revealed a highly nuanced structure–activity relationship. The alanine substitution I4A yielded a 60-fold increase in potency over the native signal –EC50 = 0.089 nM– whereas substitutions at conserved positions, D1, R3, L13, F14, abolished activity or converted the peptide into a competitive inhibitor. Importantly, these findings highlight key determinants of receptor recognition and activation unique to S. constellatus, differing notably from related streptococcal species.
Circular dichroism, CD, spectroscopy revealed that neither the native CSP nor its analogs adopted stable α-helical conformations in aqueous or membrane-mimicking environments, suggesting that secondary structure may be less critical for activity than specific side chain interactions. One exception, the R3A analog, exhibited β-sheet character and was biologically inactive – reinforcing the essential role of Arg3 in both structure and function.
Phenotypic assays confirmed that QS in S. constellatus robustly controls competence development, as measured by transformation efficiency with a spectinomycin resistance plasmid. Notably, wild-type strains were naturally competent without exogenous CSP, indicating constitutive endogenous signal production. In contrast, transformation was entirely abolished in the ΔcomC mutant, and could be rescued by exogenous CSP in a dose-dependent manner. The potent analog CSP I4A performed equivalently to native CSP, while CSP D1A effectively inhibited transformation, further validating its role as a competitive antagonist.
Contrary to observations in other streptococci, the S. constellatus QS system does not appear to regulate biofilm formation. Crystal violet assays showed no significant differences in biofilm biomass between wild-type and ΔcomC strains with or without CSP treatment, suggesting that competence and biofilm phenotypes are decoupled in this species.
This study significantly advances our understanding of QS regulation in S. constellatus. It establishes a powerful experimental framework, including a luminescence reporter, a ΔcomC mutant, and a suite of synthetic analogs for probing competence regulation at high resolution. It also reveals that S. constellatus exhibits both high natural CSP production and unusually strict structural requirements for CSP activity, distinguishing it from other streptococci. These findings may inform therapeutic strategies aimed at disrupting competence-mediated horizontal gene transfer, particularly given the clinical importance of antibiotic resistance acquisition.
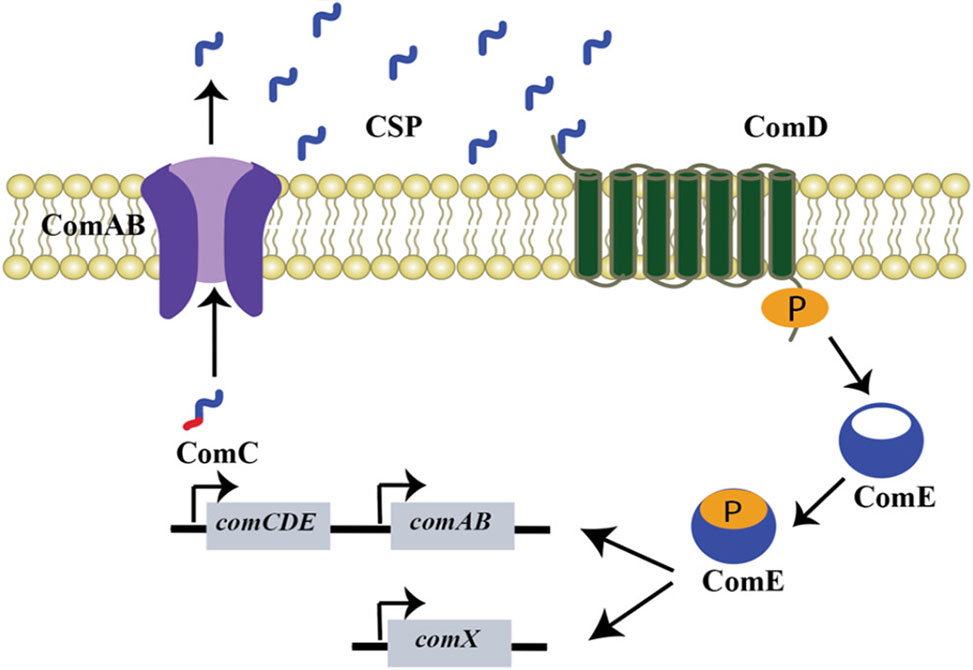
Figure 1. Illustration of the general CSP-mediated quorum sensing pathway in streptococci.

