Rational Design
Reflecting recent work in the Gellman Lab
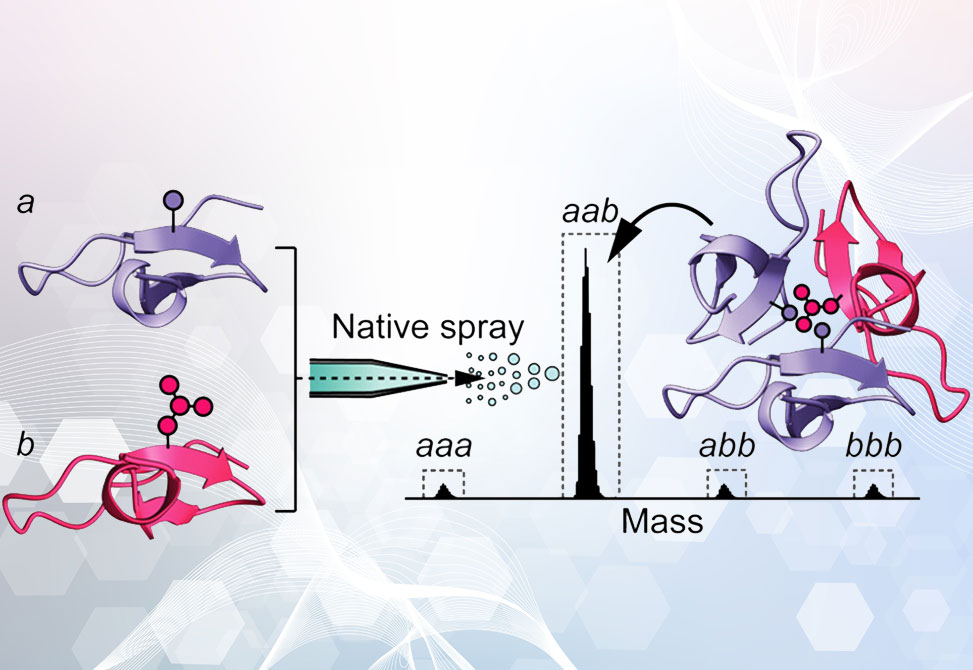
The rational construction of stable hetero-oligomeric protein complexes with defined subunit composition is a central challenge in molecular design. In this study, published in the Journal of the American Chemical Society, researchers in the Gellman Lab at the University of Wisconsin at Madison, reports the successful engineering of a highly specific aab heterotrimer derived from the well-characterized foldon homotrimer of bacteriophage T4 fibritin. By strategically destabilizing homotrimeric associations and exploiting complementary hydrophobic interactions, the authors demonstrate that quantitative native mass spectrometry, MS, can be used not merely as an analytical technique but as a decisive design tool.

The Gellman Group
The native foldon motif, a 27-residue trimeric β-propeller-like scaffold, is widely employed in vaccine and biomaterial development. Its inherent sensitivity to sequence perturbations makes it an ideal platform for engineering alternative oligomeric states. The authors initiated their design with two targeted mutations: Q11E, to introduce electrostatic repulsion between subunits and destabilize the native homotrimer, and V14A or V14L, to adjust the size of the hydrophobic core. By combining variants with either smaller, Ala, or larger, Leu, side chains at position 14, the authors hypothesized that a favorable packing interaction in a heterotrimeric configuration could be achieved.
Native MS served as the pivotal tool throughout this process, enabling precise deconvolution of monomeric, dimeric, and trimeric species and quantification of equilibrium distributions in solution. When a 2:1 mixture of G1K-Q11E-V14A and G1K-Q11E-V14L was thermocycled and analyzed by MS, a dominant aab heterotrimer population, >80%, emerged, far exceeding the statistical expectation. This compositional preference was confirmed both by gas-phase dissociation experiments and by high-resolution X-ray crystallography of crystals grown from the same 2:1 mixture. The resolved heterotrimer structure, PDB: 8UDN, maintained a foldon-like tertiary architecture with key differences in side-chain packing, supporting the hypothesis that selective assembly was driven by steric complementarity and Coulombic destabilization of competing forms.
Control experiments revealed that both the Q11E substitution and the V14A/V14L pairing were necessary for selective heterotrimer formation; removing the Q11E mutation significantly diminished the aab preference. Interestingly, crystallographic data showed that the Glu11 side chains did not participate in direct intersubunit repulsion, suggesting instead that intramolecular strain between Glu11 and Asp9 may play a destabilizing role.
This work demonstrates the remarkable utility of native MS in guiding multistep design processes for complex protein architectures. Unlike traditional structural biology techniques that require high-resolution structural snapshots, native MS offers rapid, solution-phase readouts of dynamic equilibrium states and subunit stoichiometries. By pairing this analytical precision with rational design principles, the authors provide a compelling proof-of-concept for expanding the repertoire of oligomeric protein structures beyond classical coiled-coils and triple helices.
This study establishes foldon-based heterotrimers as a novel design space for programmable protein scaffolds and exemplifies the use of native MS as a forward-looking tool for synthetic biology, molecular engineering, and biomaterial design.