Reversible Covalent Warheads
Reflecting recent work in the Gao Group
Falling in between traditional small molecules and antibodies in size, peptides are emerging as a privileged therapeutic modality, one that can harness the benefits of both small molecule and antibody drugs. To discover potential peptide therapeutics, it is highly desirable to have high throughput screening platforms that can assess peptides with diverse and non-natural functional motifs.
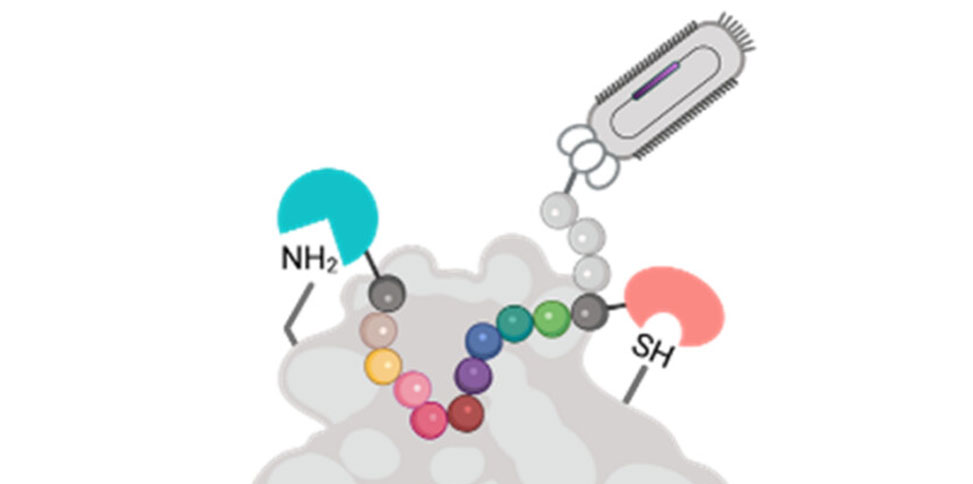
In research published in ACS Chemical Biology, researchers in the Jianmin Gao Group, present a novel phage library that incorporates two distinct designer groups. As an example, a pair of reversible covalent warheads was installed onto phage-displayed peptides to target a cysteine and a lysine. The double modification is realized by sequential modification of an N-terminal cysteine and then an internal cysteine using chemoselective chemistry. Screening of this double-warhead-presenting library against TEV protease readily revealed peptide inhibitors with single-digit micromolar potency. Importantly, the group's structure–activity studies demonstrate that both covalent warheads make important contributions to TEV protease inhibition.
The group members envision that this strategy of double phage modification can be readily extended to build phage libraries with diverse structural motifs, allowing facile expansion of the chemical space coverable by phage display.

The Gao Research Group
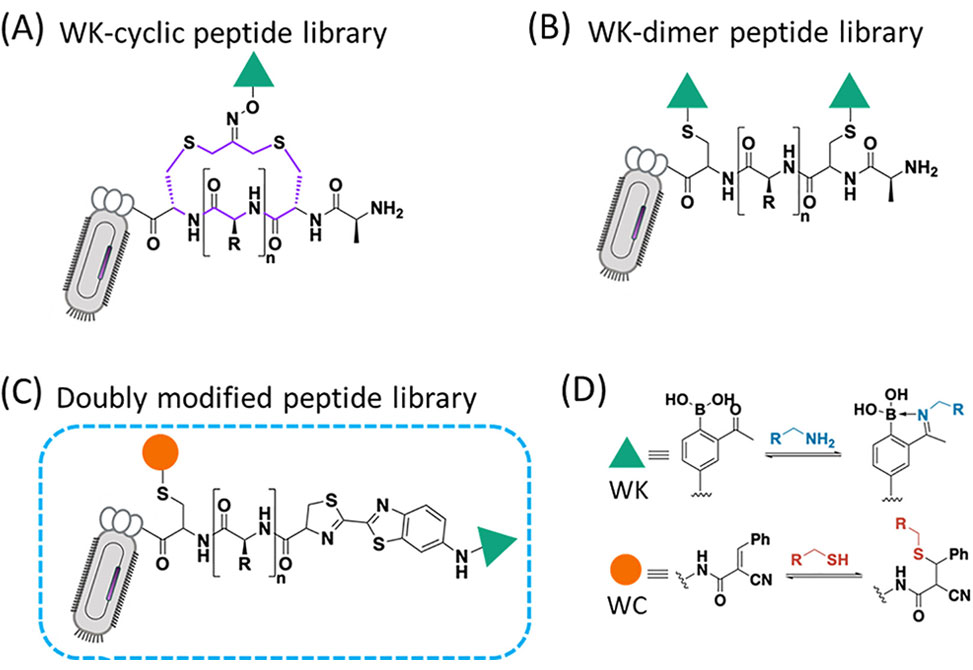 Figure 1. Phage display of reversible covalent warheads. (A) Cyclic peptide libraries displaying a lysine reversible covalent warhead (WK). (B) Lysine reversible covalent warhead (WK)-dimer library. (C) Phage peptide library displaying a lysine reversible covalent warhead (WK) and cysteine reversible covalent warhead (WC). (D) Illustration of covalent binding of amine and thiol.
Figure 1. Phage display of reversible covalent warheads. (A) Cyclic peptide libraries displaying a lysine reversible covalent warhead (WK). (B) Lysine reversible covalent warhead (WK)-dimer library. (C) Phage peptide library displaying a lysine reversible covalent warhead (WK) and cysteine reversible covalent warhead (WC). (D) Illustration of covalent binding of amine and thiol.

