Ribosomal synthesis
Reflecting recent work in the
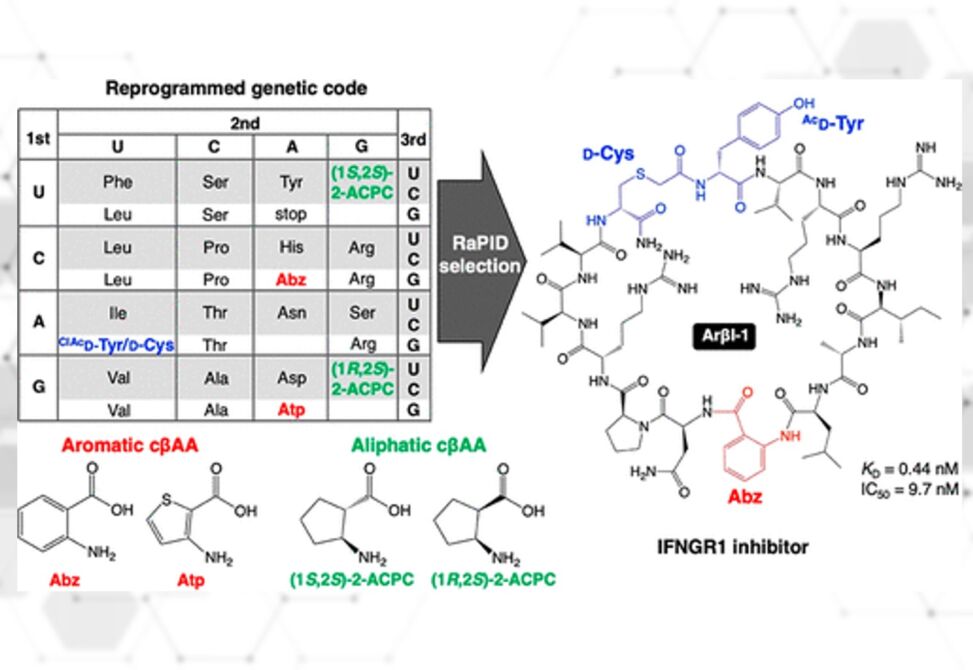
Aromatic cyclic β2,3-amino acids (cβAAs), such as 2-aminobenzoic acid and 3-aminothiophene-2-carboxylic acid, are building blocks that can induce unique folding propensities of peptides. Although their ribosomal elongation had been a formidable task due to the low nucleophilicity of their amino groups, Suga et al have recently overcome this issue by means of an engineered tRNAPro1E2 that enhances their incorporation efficiency into nascent peptide chains.
Here they report ribosomal synthesis of a random macrocyclic peptide library containing aromatic and aliphatic cβAAs, and its application to de novo discovery of binders against human IFNGR1 and FXIIa as model targets. The potent binding peptides showed not only high inhibitory activity but also high protease resistance in human serum.
Moreover, these cβAAs play a critical role in exhibiting their properties, establishing a discovery platform for de novo foldamer-like macrocycles containing such unique building blocks.