Structure Transitions
Reflecting work in the Martin and Panwar Labs
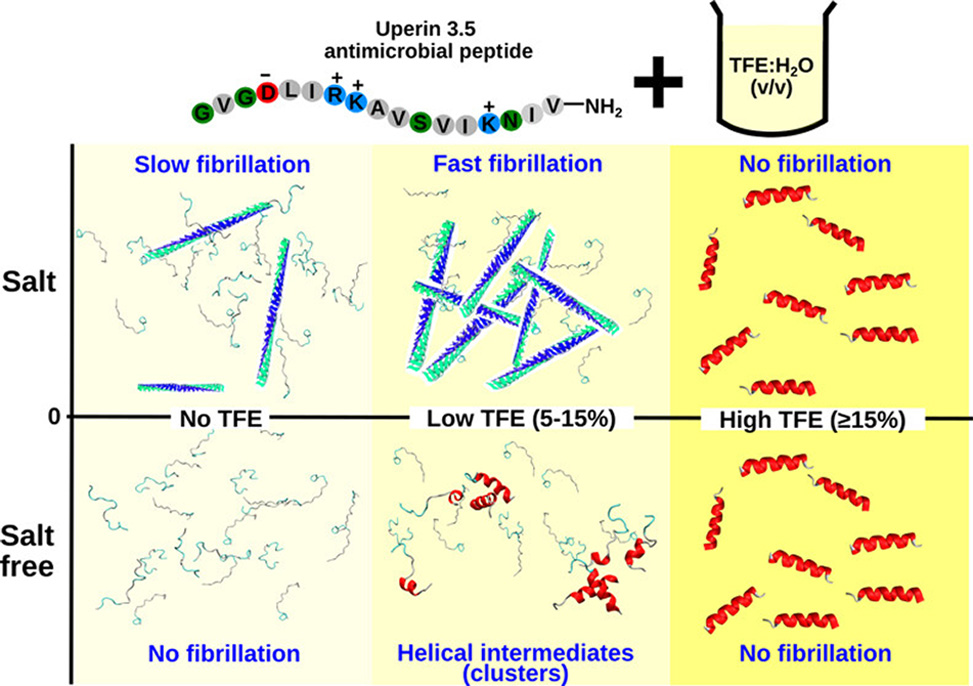
Membrane–peptide interactions are key to the formation of helical intermediates in the early stages of amyloidogenesis. Aqueous solutions of 2,2,2-trifluoroethanol, TFE, provide a membrane-mimetic environment capable of promoting and stabilizing local peptide interactions. Uperin 3.5, U3.5, a 17-residue and amidated antimicrobial peptide, is unstructured in water but self-assembles into fibrils in the presence of salt.
In a collaborative work, published in The Journal of Physical Chemistry B, members in the Martin and Panwar groups, from Monash University and Indian Institute of Technology in Bombay, respectively, investigated secondary structure transitions linked to U3.5 self-assembly in TFE/water mixtures, in both the absence and presence of salt, to assess the role of membrane–peptide interactions on peptide self-assembly and amyloid formation.
The article writers, from left to right, Lisa Martin, Ajay Panwar, Anup Prasad, and Reeju Samajdar
A 5-to-7-fold increase in fibril yield of U3.5 was observed at low TFE concentrations, 10% TFE/water v/v, compared with physiological buffer but only in the presence of salt. No aggregation was observed in salt-free TFE/water mixtures. Circular dichroism spectra showed that partial helical structures, initially stabilized by TFE, transitioned to β-sheet-rich aggregates in a saline buffer.
Molecular dynamics simulations confirmed that TFE and salt act synergistically to enhance peptide–peptide interactions, resulting in β-sheet-rich U3.5 oligomers at low TFE concentrations. Specifically, TFE stabilized amphipathic, helical intermediates, leading to increased peptide–peptide attraction through hydrophobic interactions. The presence of salt further enhanced the peptide–peptide interactions by screening positively charged residues.
Thus, this study revealed the role of a membrane mimic in stabilizing helical intermediates on the pathway to amyloid formation in the antimicrobial U3.5 peptide.