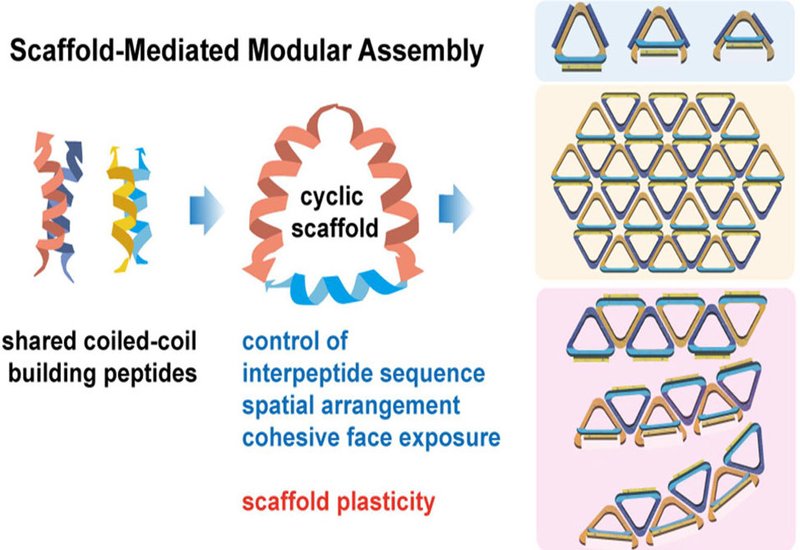
Peptides capable of folding into defined secondary structures serve as promising modular building blocks for supramolecular materials. However, conventional approaches to peptide-based self-assembly are often constrained by environmental sensitivity, polymorphic behavior, and limited structural control. In a study published in the Journal of the American Chemical Society, researchers from the Binju Wang and Tao Jiang groups at the College of Chemistry and Chemical Engineering at Xiamen University, China, introduce a robust scaffold-mediated strategy that overcomes these limitations by covalently organizing coiled-coil peptides into trifaceted cyclic scaffolds, enabling programmable and predictable assembly into diverse nanostructures.
The authors constructed cyclic trimers comprising orthogonal coiled-coil dimers—23-mer peptides, 1 & 2, and 30-mer peptides, 3 & 4, linked via disulfide and CuAAC-mediated cross-links. The resulting scaffold S1,3,3 displayed three outward-facing binding interfaces and preserved the helical conformation and specificity of its component dimers, as confirmed by CD, SEC, SV-AUC, and MALDI-MS analyses. Upon coassembly with matching peptides 2 and 4, scaffold S1,3,3 formed a discrete triangular nanostructure, S12,34,34, validated by synchrotron SAXS, ab initio shape reconstructions, DAMMIF, GASBOR, and site-specific AuNP labeling imaged by TEM. The measured geometric parameters were in excellent agreement with theoretical models and all-atom MD simulations.
By combining complementary scaffolds with varied peptide orientations, S1,3,3 and S2,4,4, the authors achieved 2D lamellar assemblies. SAXS, AFM, and TEM analyses revealed the gradual transformation from ribbons to monolayer sheets over time, driven by directional stability differences between edge pairs, 3/4 vs. 1/2. MD simulations further supported the observed assembly anisotropy.
To tune dimensionality and curvature, the team selectively blocked one edge with a free peptide, for example peptide 2, to favor 1D fibril formation. Incorporating modified scaffolds, M1,3′,3′ and L1,3″,3″, in which the cross-linking positions were progressively shifted toward the interior of peptide 3, induced controlled curvature in fibrillar assemblies. TEM, AFM, and MD analyses demonstrated increasing curvature values from scaffold S, straight, to M, moderate, to L, high curvature, with Förster resonance energy transfer, FRET, confirming corresponding conformational changes.
Molecular dynamics simulations revealed that scaffold plasticity plays a critical role in adaptive assembly behavior. Scaffold flexibility, primarily encoded in linker regions, allowed conformational expansion or contraction depending on assembly context. The ability to adopt planar or contracted shapes enabled the same scaffold to participate in structurally distinct assemblies while maintaining directional binding fidelity.
This work establishes scaffold-guided coiled-coil assembly as a powerful and generalizable method to direct nanoscale structure formation under uniform conditions. The predictability, modularity, and conformational adaptability of these trifaceted scaffolds represent a conceptual advance in peptide nanotechnology. Beyond triangular and sheet morphologies, this platform can be extended to higher-order architectures through orthogonal peptide integration, hierarchical design, or the incorporation of other bio- or synthetic macromolecular motifs. These findings open new possibilities for rational design of complex, functional biomaterials with controlled geometry and emergent properties.