Short Peptides
Reflecting work in the
Helical secondary and tertiary motifs are commonly observed as binding epitopes in natural and engineered protein scaffolds. While several strategies have been described to constrain α-helices or reproduce their binding attributes in synthetic mimics, general strategies to mimic tertiary helical motifs remain in their infancy.
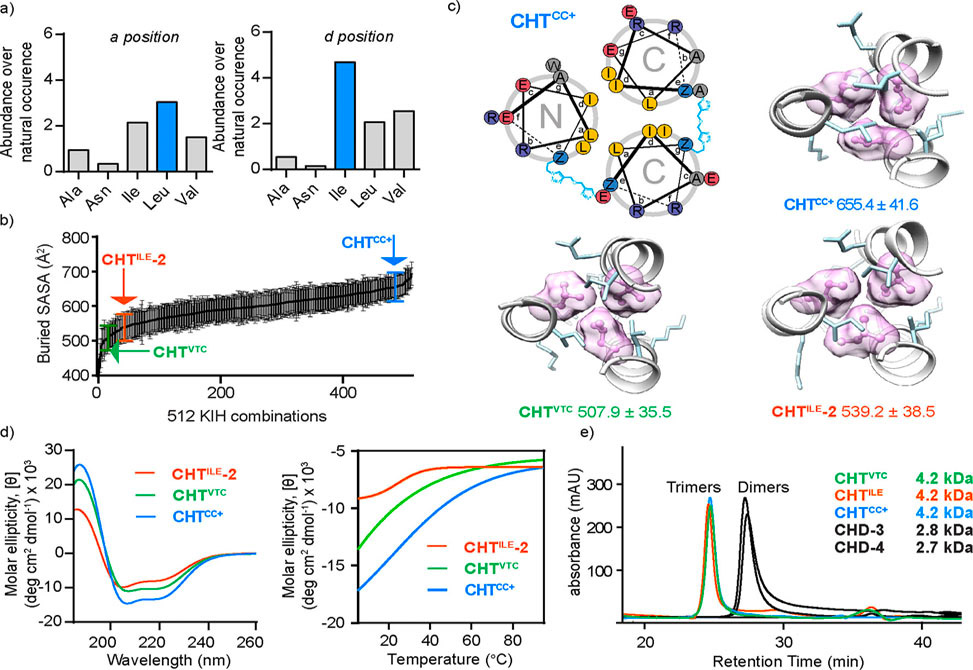
Scientists in the Arora Group at New York University, published in JACS, recently described a synthetic strategy to develop helical dimers. In that work, they found that replacement of an interhelical salt bridge with a covalent bond can stabilize antiparallel motifs in short sequences.
Now, adain published in JACS, they show that the approach can be generalized to obtain antiparallel and parallel dimers as well as trimer motifs. Helical stabilization requires judiciously designed cross-linkers as well as optimal interhelical hydrophobic packing. The group members anticipate that these mimics would afford new classes of modulators of biological function.