Solid-Phase Synthesis
Reflecting work in the Kodadek Lab
Many disease-causing proteins lack deep, readily ligandable pockets. Macrocyclic peptides and peptidomimetic compounds are one class of molecules that have shown promise in engaging these difficult targets. Macrocyclization limits the conformational flexibility of a molecule, often resulting in higher affinity binding to proteins and RNA targets of interest. In the case of peptide ligands, macrocyclization provides opportunities for the formation of intramolecular hydrogen bonds that can have profound effects on cell permeability and bioavailability. Therefore, the development of new methods for the efficient creation of macrocycles from linear precursors is an important goal. This is particularly true for the synthesis of libraries of macrocycles using diversity-oriented synthesis or split and pool strategies.
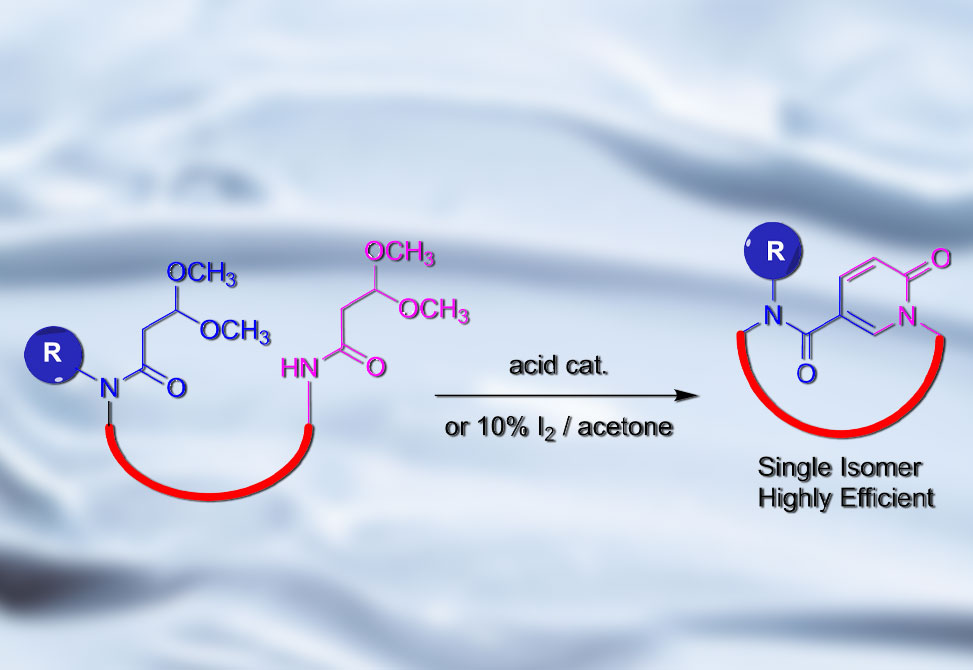
A study from the Kodadek Lab at the Scripps Institute for Biomedical Innovation and Technology, published in Jacs Au, introduces a novel solid-phase macrocyclization method generating 2-pyridone rings. The method relies on the intramolecular condensation between secondary and tertiary dimethoxy-propionic amide units, a selective reaction which leads to the formation of a single well-defined regioisomer. This method demonstrates remarkable efficiency in producing diverse peptidic and nonpeptidic bioactive targets, paving the way for the development of innovative macrocycle libraries featuring the 2-pyridone unit.
Compared to traditional peptide macrocyclization methods, the 2-pyridone linkage offers significant advantages. It requires fewer steps, achieving a high efficiency under mild conditions, making it simpler to implement. Unlike amide bond formation, which can suffer from intermolecular dimerization and necessitates high dilution, the 2-pyridone method is particularly suitable for smaller macrocycles like tetrapeptides. DMPA condensation exhibits a high selectivity, leading to high yields with minimal intermolecular side products. These findings collectively highlight the robustness and efficiency of the 2-pyridone linkage for peptide macrocyclization.