Solid Polymer Electrolytes
Reflecting work in the Evans Lab
Ion transport is essential to energy storage, cellular signalling and desalination. Polymers have been explored for decades as solid-state electrolytes by either adding salt to polar polymers or tethering ions to the backbone to create less flammable and more robust systems. New design paradigms are needed to advance the performance of solid polymer electrolytes beyond conventional systems.
In work published in Nature Materials, researchers in the Christopher Evans group at the Universwity of Illinios, highlights how the role of a helical secondary structure is shown to greatly enhance the conductivity of solvent-free polymer electrolytes using cationic polypeptides with a mobile anion.
Longer helices lead to higher conductivity, and random coil peptides show substantially lower conductivity. The macrodipole of the helix increases with peptide length, leading to larger dielectric constants. The hydrogen bonding of the helix also imparts thermal and electrochemical stability, while allowing for facile dissolution back to monomer in acid. Peptide polymer electrolytes
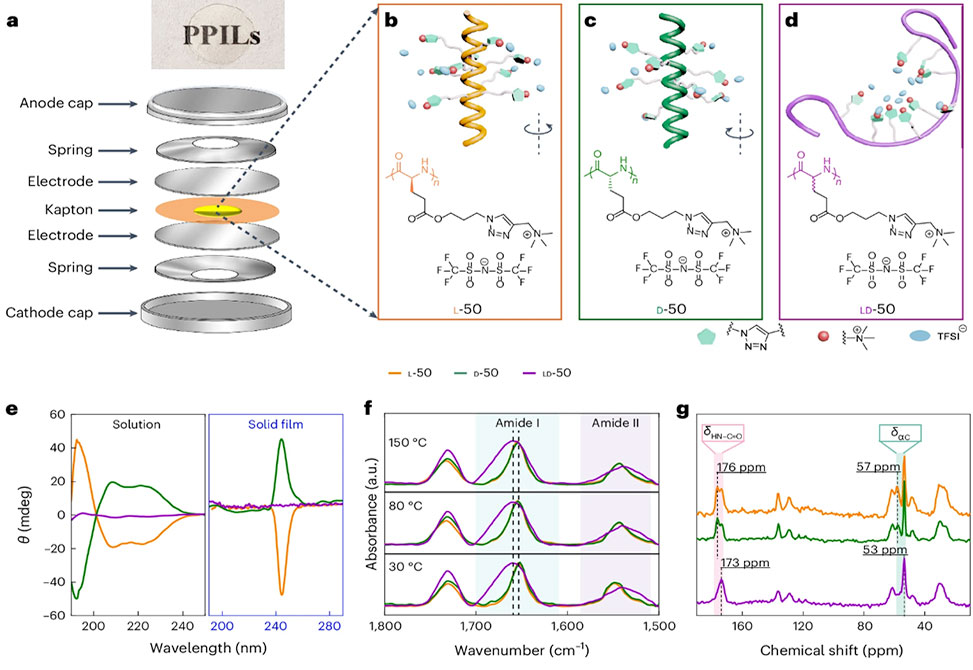
a, Schematic illustration of coin cell assembly and an image of a transparent PPIL hot-pressed film. b–d, Illustration of the secondary structure and chemical structure of PPILs, x-50s: L-50 adopted a right-handed α-helical conformation (b); D-50 exhibited a left-handed helical conformation (c); and LD-50 displayed a random coil conformation (d). e, CD spectra of x-50s in solution, 0.5mg ml−1 in methanol, and solid films. f, ATR-FTIR spectra of x-50s at different temperatures with equilibration times of 10 min. The vertical lines correspond to the amide I peak for random coils, 1,661 cm−1 and the helical structure, 1,652 cm−1. g, CP-MAS 13C NMR spectra of x-50s. The different color curves correspond to L-50, orange, D-50, green and LD-50, purple.

