Spike Peptide Amyloids
Reflecting work in the Legleiter Lab
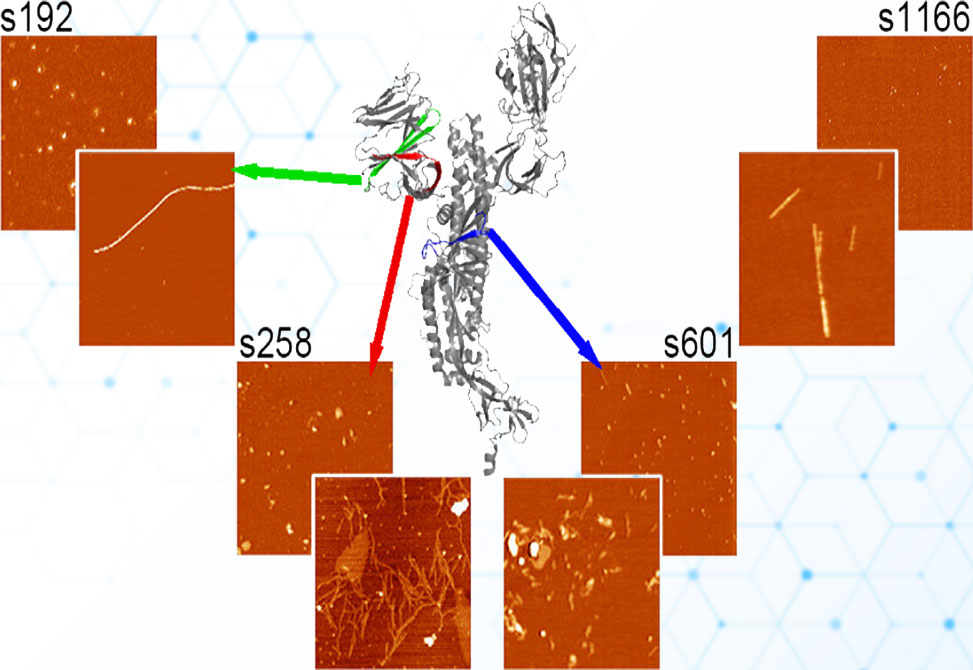
SARS-CoV-2 spike protein fragments are known to form amyloid fibrils, but far less is understood about the prefibrillar states that emerge along the way, or how those intermediates interact with lipid membranes in ways that could drive toxicity. In a study published in Biochemistry, researchers in the Legleiter lab at the University of Nevada at Reno, examine four spike-derived peptides, s192, s258, s601, and s1166, under carefully controlled, seed-free conditions to map their aggregation kinetics, catalogue oligomer and fibril morphologies, and determine how those species engage model brain-lipid bilayers. Together, the data show that aggregate behavior is not a simple function of hydropathy or net charge but instead reflects a nuanced interplay among sequence patterning, aggregation rate, and the chemistry and mechanics of the surface the peptides encounter.
Peptide stocks were HFIP-treated to erase pre-existing seeds, reconstituted, and diluted into TRIS/NaCl buffer at 50 μM, with aggregation followed by ThT fluorescence at 37 °C. Early and late time points were visualized by AFM on mica to quantify oligomer heights, diameters, and volumes, and to define fibril architectures. To probe membrane effects, the authors combined ThT assays in the presence of total brain lipid extract, TBLE, vesicles with in situ AFM on supported TBLE bilayers, imaging the same patch of membrane over time, both without and with Ca2+, which is known to stiffen anionic membranes by tightening headgroup packing. As a counterpoint to hydrophilic headgroup surfaces, in situ AFM on highly ordered pyrolytic graphite, HOPG, assessed interactions with a hydrophobic substrate.
All four peptides formed ThT-positive amyloid, but with distinct kinetic fingerprints: s1166 and s601 nucleated rapidly, s258 showed intermediate behavior, and s192 aggregated most slowly. AFM revealed correspondingly diverse oligomer populations and fibril morphologies. s192 produced a broad continuum of oligomers that matured into long, ~5–7 nm-high fibrils; s258 yielded two discrete oligomer classes that later gave thin, highly branched, laterally associating fibrils; s601 displayed early protofibrils consistent with fast nucleation and then two fibril families, raft-like ~1.5 nm structures and thicker 5.5–8 nm branched fibers, and s1166 produced sparse, small oligomers early and abundant fibrils later, with clear evidence of twisting and thick–thin interconversion. The presence of TBLE vesicles modestly delayed fibrillization for s192, s258, and s601 but not s1166, and DLS showed no vesicle fusion or flocculation, indicating that bulk lipid does not catastrophically remodel under these conditions.
Strikingly, only s192 robustly engaged and damaged supported TBLE bilayers. In the absence of Ca2+, s192 oligomers appeared within minutes, accumulated in number, and grew in height and lateral extent while the membrane roughened and disrupted over large areas. Adding Ca2+ tempered this behavior, reducing both the fraction of the surface affected and the roughness amplitude, consistent with cation-induced bilayer stiffening that raises the energetic barrier to insertion and in-plane growth. The other peptides were far less injurious: s258 and s1166 formed stable "carpets" of oligomers that neither grew appreciably nor measurably roughened the bilayer, and s601 deposited only transient, sparse oligomers with negligible coverage. Notably, all four peptides readily adsorbed to HOPG and often grew in place, underscoring how hydrophobic interfaces favor capture and expansion even when hydrophilic, anionic membranes do not.
These observations argue for a three-part mechanism. First, electrostatics and mechanics at the membrane surface gate initial capture: anionic headgroups disfavor net-negative species, and Ca2+ further tightens packing to resist insertion across the board. Second, sequence patterning—how polar and hydrophobic residues are distributed along the chain—matters more than overall GRAVY score: s192, although comparatively hydrophilic and net positive, appears "tuned" for bilayer engagement and on-surface oligomer growth, whereas more hydrophobic peptides fail to transition from surface contact to disruptive insertion. Third, aggregation state shapes toxicity windows: rapidly nucleating systems, such as s601 and s1166, may sequester into solution-phase assemblies that are less compatible with membrane insertion, while slowly nucleating s192 retains a population of surface-competent oligomers that can grow in situ and roughen the bilayer.
Methodologically, the work cautions against using bulk ThT alone to infer biological risk. Interfacial assays reveal phenotypes—stable oligomer carpets, on-surface growth, roughening without fusion that are invisible to solution kinetics yet likely decisive for cellular outcomes. Biologically, the results refine mechanistic hypotheses for post-acute sequelae: even if only a subset of spike fragments directly damages membranes, the broader ensemble of oligomers and fibrils could still perturb proteostasis, synaptic adhesion, endothelial integrity, or cross-seed host amyloids, offering multiple routes to persistent dysfunction. The limitations are clear—TBLE is a surrogate membrane lacking cell-type specificity and proteins, AFM time windows are finite, and concentrations may not mirror tissue microenvironments—but the framework is powerful. Immediate next steps include mapping sequence determinants for on-membrane growth, quantifying conductance changes to link roughening to permeability, varying lipid composition and cholesterol to mirror neuronal and endothelial membranes, and testing cross-seeding with Aβ, α-synuclein, and tau to connect these phenotypes to known disease pathways.
Author Information

Anjola Adewoye is a fourth-year Ph.D. candidate in Chemistry at West Virginia University, WVU. Her research, conducted in the Legleiter Lab under the mentorship of Dr. Justin Legleiter, focuses on understanding the behavior of amyloid-forming peptides. She investigates their capacity to form distinct aggregate species and their interactions with physiologically relevant surfaces, such as lipid membranes. By integrating experimental techniques with computational analysis, her work aims to uncover the molecular mechanisms driving amyloid aggregation and assess their potential long-term implications, ultimately informing future therapeutic strategies for amyloid-based diseases.
Anjola’s academic excellence has been recognized through the C. Eugene Bennett Fellowship in Chemistry at WVU. Beyond her lab, Anjola is committed to fostering diversity and growth within the scientific community. She regularly serves as a judge at WVU’s undergraduate research symposia, offering constructive feedback to help students strengthen their communication and research skills, particularly in STEM and chemistry. Anjola is passionate about translating scientific discovery into real-world impact and aspires to use her work to tackle complex global health and scientific challenges.

