Synthesis Tag
Reflecting work in the Hartrampf Lab
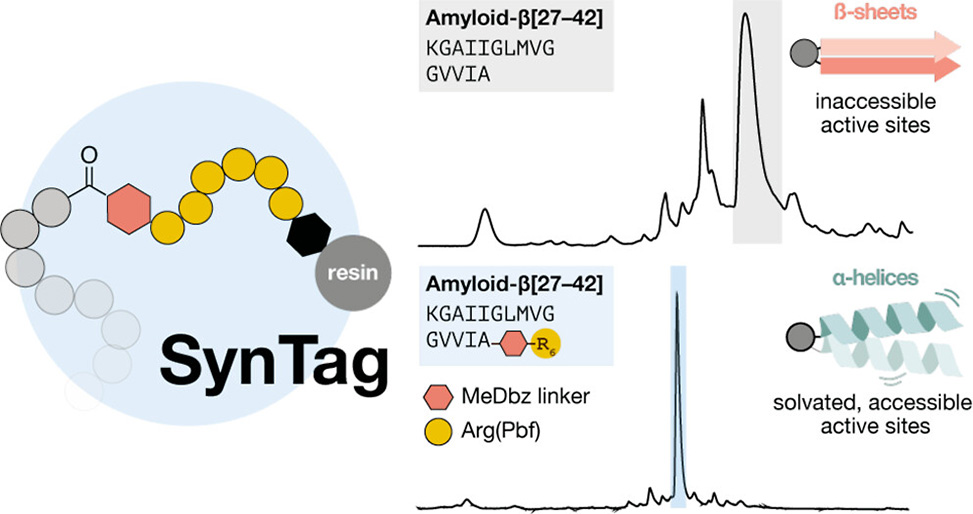
Researchers from Nina Hartrampf’s group at the University of Zürich, in collaboration with Songlin Wang at the National Magnetic Resonance Facility at Madison, NMRFAM, University of Wisconsin, have published a study in the Journal of the American Chemical Society, unveiling a novel synthesis tag, SynTag, for improving the chemical synthesis of aggregating peptides and proteins. SynTag consists of a six-arginine sequence linked to the target peptide via a cleavable MeDbz linker, offering dual benefits: mitigating sequence-dependent aggregation during solid-phase peptide synthesis, SPPS, and enhancing solubility post-cleavage.
Abstract Image
Addressing Key Challenges in Peptide Synthesis
SPPS enables the production of peptides and proteins with noncanonical amino acids and post-translational modifications, but synthesis efficiency is frequently hindered by peptide aggregation and poor solubility. The Hartrampf and Wang teams addressed these issues by incorporating SynTag into flow-based SPPS, demonstrating its ability to improve synthesis outcomes, reduce aggregation, and enhance solubility across multiple challenging peptide sequences.
Key findings include:
Aggregation Suppression: SynTag shifts the peptide conformation from β-sheet to α-helix, reducing inter- and intrachain interactions that contribute to aggregation.
Enhanced Solubility: The polyarginine tag increases hydrophilicity, facilitating HPLC purification and enabling direct hydrolysis to yield native peptide sequences.
Versatile Applications: SynTag was successfully employed in the synthesis of difficult peptides such as Barstar[75–90], amyloid-β Aβ[27–42], and the MYC transactivation domain, MYC[1–143].
Expanding the Chemical Toolbox
The study marks the first successful combination of automated fast-flow peptide synthesis, AFPS, with native chemical ligation, NCL, enabling the production of full-length MYC[1–143] via a single ligation step. Structural investigations using infrared spectroscopy, IR, and solid-state nuclear magnetic resonance, SSNMR, confirmed that SynTag prevents aggregation by disrupting β-sheet formation, improving peptide synthesis efficiency.
By providing a single, cleavable tag that addresses both aggregation and solubility, SynTag represents a transformative advance in peptide chemistry. Its broad applicability across SPPS and NCL platforms enhances the accessibility of complex peptides and proteins for biochemical and therapeutic applications.


