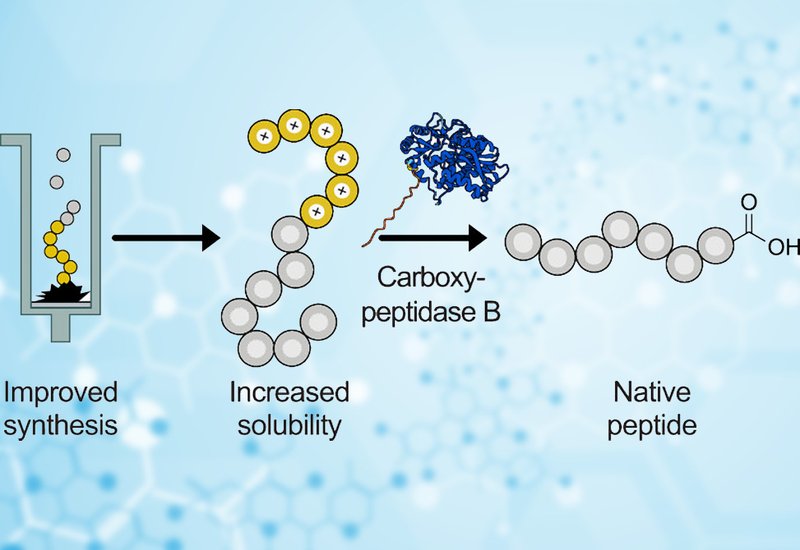
Peptide chains misbehave. During solid-phase peptide synthesis, growing chains can collapse into sticky β-sheet structures that clog the resin, block coupling reactions, and leave chemists with impure products. This aggregation problem has plagued the field for decades, forcing researchers to accept lower yields or invest in expensive specialty resins that swell better but cost more. Real-time UV monitoring on modern flow synthesizers can detect exactly when aggregation begins, yet knowing where the problem starts does not solve it. A team led by Nina Hartrampf at University of Zürich, UZH, published in ACS Chemical Biology, now offers an elegant workaround: a temporary hexaarginine tag that keeps peptide chains orderly during synthesis and then disappears on command.
The researchers built on their earlier "SynTag" concept, which attached six arginine residues to the C-terminus of growing peptides. The six Arg(Pbf) amino acid building blocks induce helical structure in the elongating chain on resin, preventing the β-sheet interactions that trigger aggregation. The original approach required a specialized MeDbz linker to remove the tag after synthesis, but this linker introduced complications. Activation sometimes failed, side products accumulated, and the extra steps added cost and complexity.
The new "ArgTag" strategy eliminates the linker entirely. Instead, the team optimized an enzymatic removal method using recombinant Carboxypeptidase B, which chews through C-terminal arginine residues under mild aqueous conditions at pH 7.8 and 37°C. The enzyme works cleanly: it removes all six arginines without touching internal residues, leaving behind the native peptide with its natural C-terminal carboxylic acid. Notably, no intermediate species appeared during digestion; the enzyme apparently grips its substrate until all basic residues are gone. When the researchers compared both approaches on GLP-1, the ArgTag route delivered 71% crude purity versus 43% for the activated SynTag, which suffered from extensive side product formation during linker activation.
The team then stress-tested their tag across six different resin types spanning the full polarity spectrum. They synthesized Barstar[75–90], a notoriously aggregation-prone 16-residue sequence, on everything from cheap polystyrene supports to premium PEG-based resins. The ArgTag improved crude purity on every single platform. On TentaGel XV-RAM, purity jumped from 45% to 72%. Even on basic polystyrene resin, where the untagged synthesis barely produced identifiable product, the ArgTag delivered 24% crude purity with a clean chromatographic peak. High-loading resins, preferred in industrial settings because they reduce solvent consumption, showed equally strong improvements. In some cases, the purity gains were even more pronounced at higher loadings.
To confirm that the approach scales beyond the laboratory bench, the researchers moved to a pilot-scale flow synthesis platform. Using just 5 equivalents of amino acid instead of the 20–40 equivalents typical of their analytical system, and working at 50°C rather than 90°C, they still achieved nearly doubled crude purity for the difficult Barstar sequence. The ArgTag performed regardless of temperature, reagent type, DIC/Oxyma vs. HATU or PyAOP, and stoichiometry, or batch size.
This work removes a practical barrier that has limited synthetic access to aggregation-prone peptides. The ArgTag requires no special linkers, functions across resin chemistries and price points, and scales to preparative quantities. For peptide chemists targeting difficult sequences, whether in academic labs pursuing chemical biology applications or manufacturing facilities producing therapeutic candidates, the approach offers a straightforward path to cleaner syntheses and higher yields. The method is particularly valuable given recent supply constraints on premium resins; the ArgTag lets researchers achieve excellent results on economical supports that previously underperformed.