Targeted Conjugates
Reflecting work in the Zeglis Group
For two decades, immunoconjugates have been a mainstay of targeted oncology, from antibody–drug conjugates to radioimmunotherapy probes. The clinical challenge has never been the underlying concept, but rather the chemistry of conjugation. Conventional strategies, stochastic coupling to lysines or other surface residues, are simple and inexpensive but yield heterogeneous mixtures with variable degrees of labeling. This molecular diversity can compromise pharmacokinetics, antigen recognition, and imaging contrast, limiting the translational potential of otherwise powerful agents. As a result, the field has been steadily shifting toward site-specific modification, leveraging unnatural amino acids, chemoenzymatic ligations, or disulfide reduction. Each approach improves homogeneity but introduces new barriers, from cost and complexity to instability of linkages. The need for a fast, robust, and translatable chemical method has been acute.
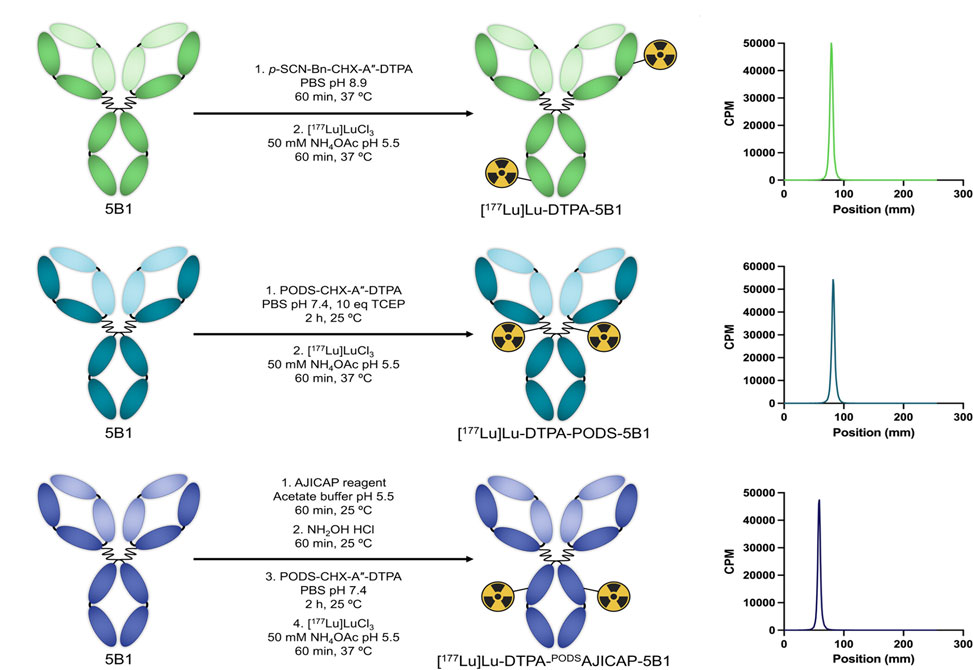
In a communication published in Bioconjugate Chemistry, researchers in the Zeglis Lab at Hunter College, introduce the AJICAP reagent, a 17-amino acid cyclic peptide that binds to a protein A recognition motif in the Fc domain of IgG1–4 antibodies and directs modification to the heavy-chain lysine K248. Once bound, its thiophenyl ester reacts with the proximal amine, and subsequent hydroxylamine treatment liberates the peptide scaffold to yield a pair of free thiols. This traceless maneuver produces a heavy-chain–specific, sulfhydryl-bearing intermediate ready for thiol-reactive cargo. In prior work, AJICAP enabled antibody–drug conjugates of trastuzumab and rituximab with controlled degree-of-labeling and excellent stability. Here, the platform is extended to the synthesis of radioimmunoconjugates by attaching a bifunctional chelator—CHX-A″-DTPA equipped with a phenyloxadiazolyl methyl sulfone, PODS, group, followed by coordination of therapeutic lutetium-177. The model antibody, 5B1, targets the carbohydrate antigen CA19-9, widely expressed in pancreatic ductal adenocarcinoma, offering a clinically relevant testbed.
The synthetic sequence proved straightforward. AJICAP treatment of 5B1, hydroxylamine cleavage, and conjugation to PODS-DTPA yielded DTPA-PODSAJICAP-5B1 in ~65% yield with a degree-of-labeling of 1.8 ± 0.1. For comparison, two controls were prepared: a disulfide-reduced site-selective conjugate, DTPA-PODS-5B1, and a conventional stochastic lysine conjugate, DTPA-5B1. All three were labeled with [177Lu]Lu3+ to high yield and radiochemical purity, and all exhibited excellent serum stability over five days. Immunoreactivity toward CA19-9 was retained, with blockable antigen binding and fractions exceeding 0.75. Importantly, autoradiography confirmed heavy-chain localization of the AJICAP modification, consistent with K248 targeting, while the other two approaches produced a broader but still predominantly heavy-chain distribution.
In vivo, each of the three radioimmunoconjugates displayed strong tumor tropism in BxPC3 pancreatic xenografts, with high and sustained uptake. At 144 h, tumoral accumulation reached 52.0 ± 24.5 %ID/g for the AJICAP construct, compared with 42.9 ± 13.7 %ID/g for the disulfide variant and 18.8 ± 5.0 %ID/g for the stochastic analogue. Tumor-to-blood ratios were broadly similar across constructs, but tumor-to-bone ratios were highest for the AJICAP probe. Statistically significant differences emerged mainly in comparisons to the stochastic conjugate, underscoring that even traditional methods can perform well when paired with a robust antibody scaffold such as 5B1. The authors note that the advantages of AJICAP may be more dramatic with less stable immunoglobulins, a hypothesis now under active exploration.
The implications are notable. AJICAP offers a purely chemical, enzyme-free, one-pot/two-step strategy for site-specific conjugation, compatible with native antibodies and modular across payloads. Unlike maleimide–thiol linkages, it avoids instability; unlike enzymatic ligations, it is rapid and low-cost. The product is homogeneous, stable, and in this case, clinically competitive. The authors foresee adaptations toward a single-step AJICAP reagent that directly installs a chelator or drug, further simplifying production and automation.
This work establishes AJICAP as a practical platform for the generation of homogeneous radioimmunoconjugates. While its superiority in vivo may be muted in the context of a highly optimized antibody, the chemistry’s reproducibility, modularity, and scalability mark it as a powerful addition to the toolkit for next-generation radiopharmaceuticals and antibody-based therapeutics.