T cells read short pathogen fragments on major histocompatibility complexes, MHC, using T cell receptors, TCR. Antigen recognition depends on the three-dimensional fit of TCR, peptide, and MHC, yet predicting which peptides will bind and activate a given TCR has been difficult. In work published in PNAS, Visani and colleagues from the University of Washington, introduce a structure-aware machine learning approach that forecasts TCR–peptide–MHC interactions and designs new immunogenic peptides that trigger T cell responses.
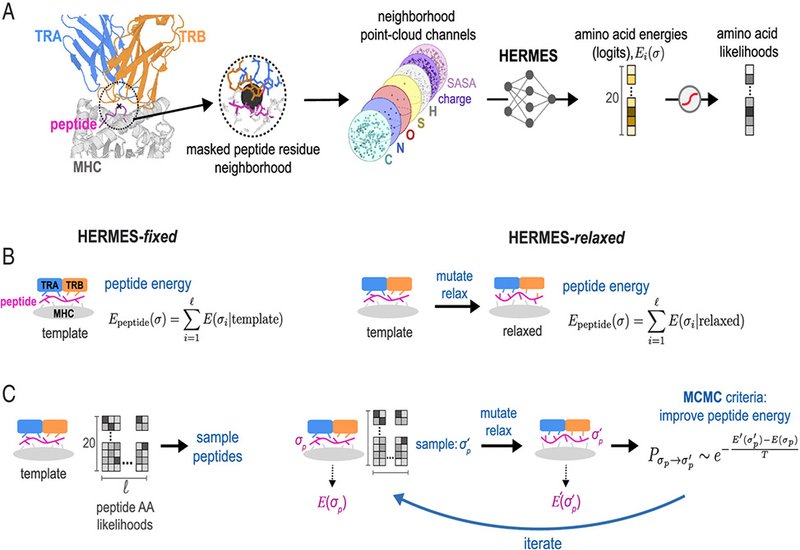
The method centers on HERMES, a physics-guided, 3D-equivariant model trained on diverse protein structures to infer amino acid preferences from local atomic environments. Without direct training on TCR–pMHC data, HERMES scores how well a candidate peptide fits inside a specific TCR–MHC groove. Two practical protocols are used: HERMES-fixed, which evaluates peptides on a high-quality template structure, and HERMES-relaxed, which permits local backbone and side-chain adjustments before rescoring. Together, these routes translate structural context into a single peptide energy that correlates with binding and activity.
Across benchmark systems, including the cancer-testis antigen NY-ESO-1 with TCR 1G4 and the viral Tax peptide with TCR A6, HERMES predictions track experimental binding affinities, Spearman correlations up to 0.72, and compare favorably with sequence-only baselines and contemporary structure-based methods. Although functional T cell readouts reflect more than affinity alone, HERMES scores still align with measured activities for multiple TCRs against cytomegalovirus and tumor epitopes, showing useful generalization when reliable structures are available.
Leveraging this recognition model, the team executes de novo peptide design for three therapeutically relevant systems, NY-ESO, EBV, and MAGE. Designed peptides differ by as many as five substitutions from native sequences, yet activate T cells at rates up to 50% overall, with higher success, about 70%, within five-mutation neighborhoods for HERMES-fixed designs. Structure–function trends emerge cleanly, for example, glutamic acid at position 8 strengthens polar contacts to the TCR in the MAGE framework and elevates activity, while fixing critical anchor residues, such as E1 to the MHC, improves success and broadens the viable search.
The study also quantifies TCR specificity by estimating the entropy of peptide preferences from template structures. Typical TCR–MHC pairs recognize on the order of 103 peptides, far fewer than the many billions that MHC molecules can present alone, highlighting how TCR contacts restrict accessible antigen shape space. AlphaFold3, AF3, templates generally preserve performance when crystal structures are unavailable, although high-quality starting models remain important for accurate ranking.
Taken together, HERMES offers a practical, structure-aware path to predict TCR recognition, design immunogenic peptides, and probe TCR degeneracy. This framework can support engineered T cell therapies and peptide vaccines by nominating potent, structurally compatible epitopes, then narrowing experimental effort to the most promising candidates while guarding against off-target liabilities.