Triceptide Biocatalysts
Reflecting recent work in the Morinaka Group
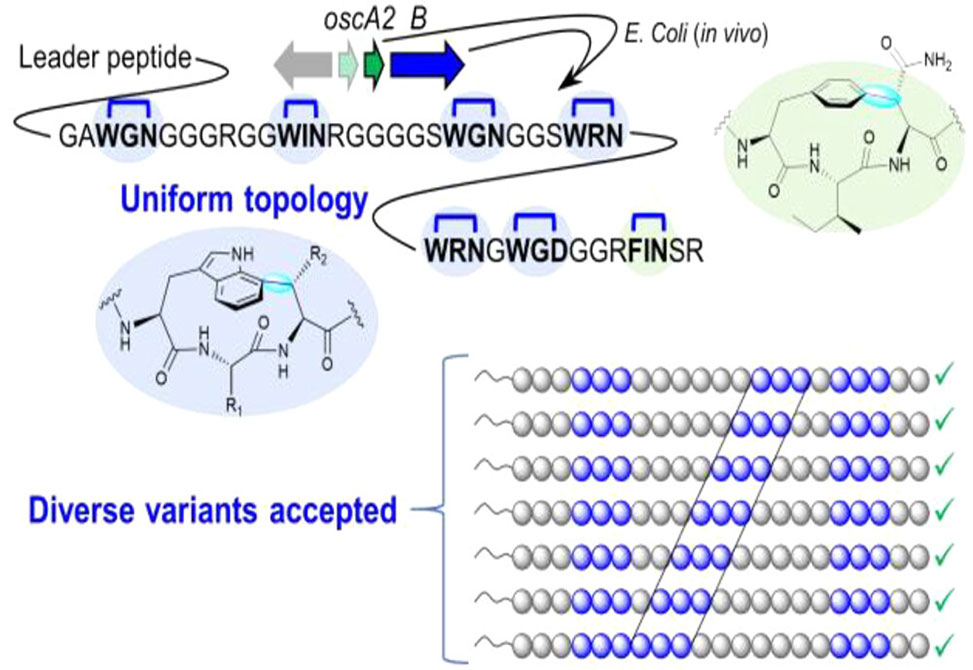
Triceptides are a class of ribosomally synthesized and post-translationally modified peptides defined by an aromatic C(sp2) to Cβ(sp3) bond. The Gly-rich repeat family of triceptide maturases, TIGR04261, are paired with precursor peptides, TIGR04260, containing a Gly-rich core peptide. These maturases are prevalent in cyanobacteria and catalyze cyclophane formation on multiple Ω1-X2-X3 motifs, Ω1 = Trp and Phe, of the Gly-rich precursor peptide. The topology of the individual rings has not been completely elucidated, and the promiscuity of these enzymes is not known.
In a study published in ACS Chemical Biology, researchers in the Morinaka Group at the National University of Singapore, characterized all the cyclophane rings formed by the triceptide maturase OscB and show the ring topology is uniform with respect to the substitution at Trp-C7 and the atropisomerism, planar chirality. Additionally, the enzyme OscB demonstrated substrate promiscuity on Gly-rich precursors and can accommodate a diverse array of engineered sequences.
These findings highlight the versatility and implications for using OscB as a biocatalyst for producing polycyclophane-containing peptides for biotechnological applications.