Catch-and-Release Peptide Purification
Peptide Purification
Peptide-related impurities can significantly influence the outcome of drug research and development, for example, false-positive results, and must be removed to ensure high assay reliability. Ultimately, such impurities must be removed entirely from active pharmaceutical ingredients to provide the highest quality clinical-grade material.
Traditional chromatographic methods mainly carry out the purification of chemically synthesized peptides. Reversed-phase high-pressure liquid chromatography, RP-HPLC, is the most commonly used method. Also, reversed-phase flash chromatography, flash, represents an established technique for research-grade peptide purifications and intermediate/clean-up purification steps of higher-grade material. Due to similar properties, however, co-eluting impurities may be overlooked with both these chromatographic techniques.
In contrast, chemo-selective separation principles drive Gyros Protein Technologies’ orthogonal purification PurePep® EasyClean technology. Capping during solid-phase peptide synthesis, SPPS, ensures that only the full-length peptide is accessible for modification with a traceless cleavable purification linker, PEC-Linker, at the end of SPPS. The target peptide can then be isolated from a complex mixture via orthogonal catch-and-release on modified beads.1,2
Moreover, optimizing individual syntheses is often impossible if researchers aim to synthesize multiple peptides simultaneously, and time is essential. Therefore, truncations and deletions become significant sources of contamination, even with high coupling efficiencies. For example, a 30-mer peptide can have more than 25% truncations despite a 99% coupling efficiency, see figure 1, below.
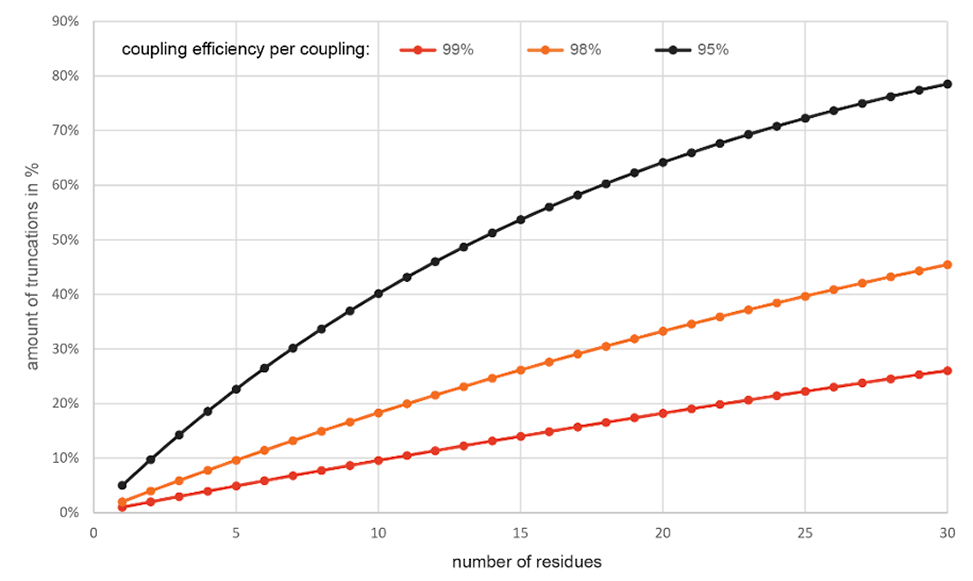
Figure 1. Amount of truncations calculated by: % = 100 – 100*(0.9X)^n-residues; X: 9, 8, 5
PurePep® EasyClean Peptide Purification
The PEC-Linker RC+ generation reflects the latest advancements in catch-and-release methodologies, Figure 2.1 All three construction blocks in the linker molecule are optimized for general applicability and a reliable and highly efficient purification and modification experience.
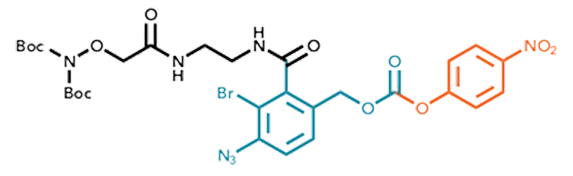
Figure 2. Molecular structure of the PEC-LInker RC+.
1. After removing the Boc protecting group during the acid treatment for cleavage from the SPPS resin
2. A Bromo-substituted para azido-benzyl carbamate acts as the cleavable unit and represents the heart of the PEC-Linker RC+. The construction enables a well-balanced stability behavior, depending on the medium’s pH: Reducing the azide to an amine sensitizes the linker to cleavage. However, the fracture does not occur at neutral pH, enabling the washout of by-products formed during reduction. Finally, treating the safety-release system with weak acids liberates the peptide through an acid-catalyzed 1,6-elimination.
3. The para-nitrophenol represents an ideal leaving group with precisely tuned reactivity and storage stability.
How Catch-and-Release Purification Works
The general scheme of PEC purification by catch-and-release consists of six steps shown below.
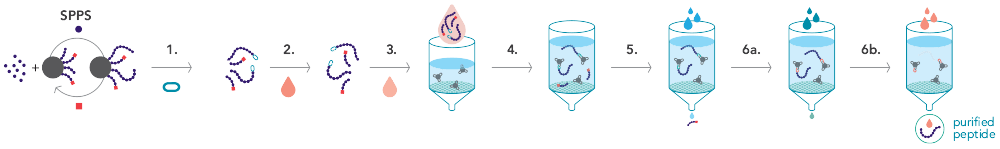
Figure 3. Detailed scheme of the PEC process using the PEC-Linker RC+.
1. Couple the PEC-Linker to the target peptide at the end of the solid-phase peptide synthesis, SPPS. Capping after each amino acid coupling cycle to ensures the selective coupling on the target full-length sequence.
2. Cleave the peptide from the SPPS resin using respective TFA-cocktails.
3. Immobilize through covalent capture, “Catch,” on the activated filter material in an oxime ligation.
4. Covalent capture allows the washing out of unbound substances such as truncated sequences and enables selective modification of the bound unprotected peptide.
5. Precipitate and dissolve the peptide.
6. a) The subsequent reduction of the PEC-Linker sensitizes the system for-safety release of the peptide.
b) Liberate the purified peptide via weak acidic induced 1,6-elimination and elution, “Release.”
A PEC Purification Case Study
The purification of the hydrophilic peptide Histone H3, (1-20; H-ARTKQ TARKS TGGKA PRKQL-OH), synthesized via SPPS, is a formidable challenge due to co-eluting impurities. In this example, we show how to achieve higher purities and lower solvent consumption using PurePep EasyClean Starter Kits and RP-HPLC in a simple orthogonal purification approach.
In this case study, we demonstrate the orthogonality of PEC with RP-HPLC by purifying the polar fragment of the Histone H3 (1-20) peptide using the Starter Kit.
PEC-Grade Peptides
The UV-chromatogram of the crude Histone H3 (1-20) appears very clean at first glance, Figure 4, left. However, several capped deletion sequences assemble in the mass analysis of the corresponding peak, inset of Figure 4, left. Major impurities were Ala (A)-, Arg (R)-, and Thr (T) deletions. An analytical HPLC run with 0.1% HFBA resolved the underlying peaks, and a crude purity of 29% was determined, chromatogram is not shown.
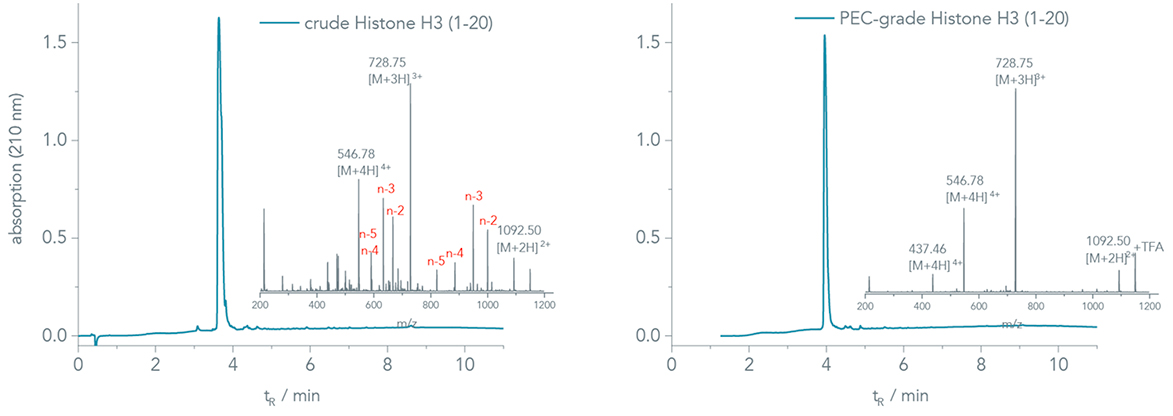
Figure 4. UV chromatograms, 210 nm, for the crude and PEC-grade Histone H3 (1-20) fragment.
Figure 4, right, shows the chromatogram of the PEC-grade peptide. A closer look at the mass spectra, insets in Figure 4, right, reveals that PEC efficiently removes the co-eluting impurities in a single step, ensuring high assay reliability.
Solvent Economy
Chromatographic separation consumes large amounts of organic solvents. Therefore, reducing solvent use – one of the 12 principles of Green Chemistry – is critical to improving the ecological footprint of peptide purification.
ACN consumption is a reasonable measure to evaluate the ecological benefit of the orthogonal approach. Due to PEC‘s catch-and-release principles, users work with a small amount of organic solvents and produce less waste—only 50 mL of ACN and 200 mL of total waste accumulate during the process.
In contrast, the flash purification required 500 mL MeCN and produced 1500 mL total solvent, resulting in an overall saving of more than 85% of solvent use and waste.
Learn more details in the Application Note about PEC peptide purification for reducing solvent consumption.
High-Throughput PEC Purification
The principle of catch-and-release purification can easily be transferred to various formats, such as fritted well plates for high-throughput purification.
Download the application note about a one-method-fits-all procedure that creates PEC-grade peptide libraries with an average purity of 86% in a 96-well filter plate format.

The PurePep® EasyClean Starter Kit
Our Starter Kit works as the perfect introduction to the world of PEC peptide purification. The Ready-to-Use package comes with PEC-Linker, cartridges pre-filled with activated filter material, and related consumables. You can choose between two formats to provide you with the ideal kit in a suitable number and scale for your purification needs.
Starter Kit 8 x 25 μmol*: meet your research demand
Starter Kit 8 x 100 μmol*:tackle the elevated scales
*referring to synthesis scale
Highlights
- The simplest access to PEC-grade peptides – works even with manual setup
- Purified peptides at the most common R&D scales from 25 to 100 µmol – up to 200 mg of final product per syringe
- Fastest access to 8 or more purified peptides per day
- Low solvent consumption and waste generation – 75% lower than traditional methods
Note: More consumables are required to run the purification. Please read the Gyros FAQ for more information.