Lab of the Month – The Schmeing Lab
Research in the Schmeing Lab
The Schmeing Lab focuses on understanding how large, natural biosynthetic enzymes create their remarkable and valuable products. Two key enzyme systems under investigation are nonribosomal peptide synthetases, NRPSs, which synthesize important compounds such as the antibiotic daptomycin, the anti-cancer drug actinomycin, and the immunosuppressant cyclosporin, and cyanophycin synthetase, which produces a versatile and sustainable green polymer.

Using cutting-edge techniques including cryo-electron microscopy, cryo-EM, X-ray crystallography, chemical biology, and biochemical approaches, our lab visualizes and deciphers the complex mechanisms of these enzymes. This research not only provides a deeper understanding of these natural nano-factories but also enables bioengineering advancements for the synthesis of novel therapeutics and environmentally friendly chemicals.
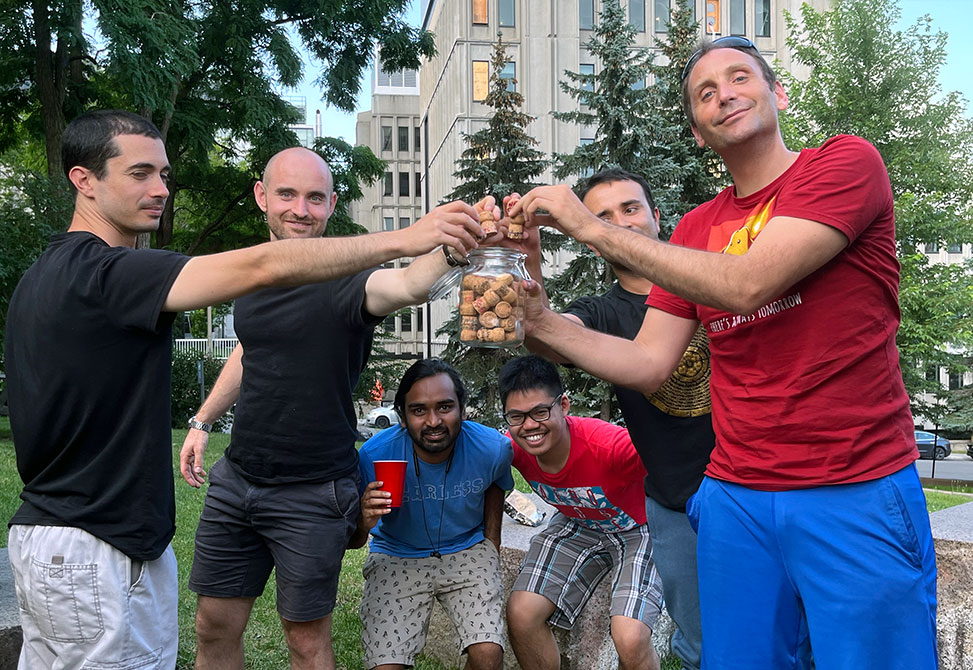
In addition to scientific discoveries, we bring these systems to life through molecular animations that illustrate intricate biosynthetic processes. These animations have become a hallmark of our presentations at conferences and seminars, often paired with dynamic music to engage audiences. If you’ve seen a visual explanation of an NRPS, cyanophycin synthetase, or ribosome in action, it was likely our work!
Who We Are
The Schmeing Lab is a dynamic and collaborative team that includes M.Sc. students, Ph.D. students, postdoctoral fellows, and a research associate, supported by undergraduate researchers who join through independent study courses or internships. Our team has welcomed members from over 20 countries across five continents, reflecting the global nature of science and creating a rich multicultural environment.

This diversity brings fresh perspectives to our work, fostering creativity, innovation, and a supportive atmosphere. We not only share scientific ideas but also take the time to learn from each other’s cultures, making the lab a uniquely enriching and collaborative space.
A Broader Network
The Schmeing Lab is part of the Centre for Structural Biology, where Martin serves as Director. This affiliation provides access to state-of-the-art facilities, interdisciplinary collaboration opportunities, technical training, and professional development workshops. The Centre also hosts research-in-progress talks (with free pizza!) and supports travel grants, offering a comprehensive ecosystem for growth and success.
Within the Biochemistry Department, we are surrounded by researchers tackling diverse challenges in molecular biology, creating exciting opportunities for collaboration. These interactions often lead to new ideas, shared expertise, and impactful publications.

Where We Work and Play
Our lab is housed in the Bellini Life Sciences Building, situated in the heart of downtown Montreal. This prime location combines the vibrant energy of a cosmopolitan city with the serene beauty of Mount Royal, a leafy park ideal for jogging, cross-country skiing, or simply enjoying nature between experiments.

But we don’t just work hard—we also know how to have fun. Some of our cherished traditions include:
-
Annual Fun Weekend: A three-day canoe-camping trip in the Canadian wilderness, where we bond as a team and search for the elusive “Batmoose,” our lab’s spirit animal.
-
Celebratory Cork Drops: Marking the publication of new papers by dropping corks in a unique tradition.
-
Lab Game Days: Using games like brainball to creatively name new equipment and hosting fun, team-building activities.
-
Weekly Rituals: Enjoying Montreal’s famous bagels every Friday and well-earned coffee breaks to recharge.
-
Holiday Dinner: A festive evening featuring delicious food and the competitive Danish dice gift exchange.
Our commitment to fostering a positive, collaborative environment extends beyond the lab bench, ensuring that everyone feels supported and inspired to achieve their best.
To learn more about our research, team, and activities, visit our lab website.
Martin Schmeing received his Bachelor of Science from McGill University, followed by a Ph.D. under the mentorship of Nobel laureate Tom Steitz at Yale University. After completing his doctoral studies, he pursued postdoctoral research with another Nobel laureate, Venki Ramakrishnan, at the Laboratory of Molecular Biology in Cambridge.
In 2010, Martin returned to McGill University, where he currently holds the title of James McGill Professor in the Department of Biochemistry and serves as Director of the Centre de recherche en biologie structurale. His laboratory focuses on elucidating the structure and function of nonribosomal peptide synthetases, NRPSs, and other peptide-synthesizing macromolecular machines. These large microbial enzymes are notable for catalyzing the synthesis of peptides via amide bond formation between monomeric building blocks, typically amino acids.
The products of NRPSs often exhibit remarkable chemical and biological properties, making them valuable as therapeutics—antibiotics, antivirals, anti-cancer agents, and immunosuppressants—and as natural, sustainable chemicals such as emulsifiers, siderophores, and research tools.
Martin’s groundbreaking work in structural biology has garnered numerous accolades, including:
-
The Young Investigator Award from the Canadian Society for Molecular Biosciences
-
The Joe Doupe Award from the Canadian Society for Clinical Investigation
-
The Bhagirath Singh Early Career Award from the Canadian Institutes of Health Research
-
A Career Development Award from the Human Frontier Science Program Organization
-
A Canada Research Chair
-
The Biological & Medicinal Chemistry Lectureship Award from the Chemical Institute of Canada
Through his innovative research and commitment to understanding macromolecular machines, Martin continues to advance the frontiers of structural biology, contributing to both fundamental science and the development of applications that address global challenges.
Select Publications
-
Pistofidis, A., Ma, P., Li, Z., Munro, K. A., Houk, K. N., & Schmeing, T. M.
Structures and mechanism of condensation in nonribosomal peptide synthesis. Nature. https://doi.org/10.1038/s41586-024-08417-6 -
Folger, I. B., Frota, N. F., Pistofidis, A., Niquille, D. L., Hansen, D. A., Schmeing, T. M., & Hilvert, D. (2024). High-throughput reprogramming of an NRPS condensation domain. Nature Chemical Biology, 20(6), 761–769.
-
Sharon, I., McKay, G., Nguyen, D., & Schmeing, T. M. (2023). Discovery of cyanophycin dipeptide hydrolase enzymes suggests widespread utility of the natural biopolymer cyanophycin. Proceedings of the National Academy of Sciences of the United States of America, 120(8), e2216547120.
-
Patteson, J. B., Fortinez, C. M., Putz, A. T., Rodriguez-Rivas, J., Bryant, L. H., Adhikari, K., Weigt, M., Schmeing, T. M., & Li, B. (2022). Structure and function of a dehydrating condensation domain in nonribosomal peptide synthesis. Journal of the American Chemical Society, 144(31), 14057–14070.
-
Sharon, I., Haque, A. H., Grogg, M., Lahiri, I., Seebach, D., Leschziner, A. E., Hilvert, D., & Schmeing, T. M. (2021). Structures and function of the amino acid polymerase cyanophycin synthetase.
Nature Chemical Biology, 17, 1101–1110. -
Reimer, J. M., Eivaskhani, M., Harb, I., Guarné, A., Weigt, M., & Schmeing, T. M. (2019). Structures and bioengineering of a dimodular nonribosomal peptide synthetase. Science, 366(6466).
-
Huguenin-Dezot, N., Alonzo, D. A., Heberlig, G. W., Mahesh, M., Nguyen, D. P., Dornan, M. H., Boddy, C. N., Schmeing, T. M., & Chin, J. (2019). Trapping biosynthetic acyl-enzyme intermediates with encoded 2,3-diaminopropionic acid. Nature, 565(7737), 112–117.
-
Reimer, J. M., Aloise, M. N., Harrison, P. M., & Schmeing, T. M. (2016). Synthetic cycle of the initiation module of a formylating nonribosomal peptide synthetase. Nature, 529(7585).



