Lab of the Month – Sun Group
Who We Are
The Hao Sun Research Group at the College of Sciences, Nanjing Agricultural University, pioneers chemical protein synthesis to generate challenging targets, including post-translationally modified variants such as ubiquitinated and phosphorylated proteins. These synthetic tools enable detailed structural, biochemical, and functional studies, opening new opportunities in protein science.

Professor Hao Sun’s Postdoctoral Advisor and 2025 APS du Vigneaud Awardee, Professor Ashraf Brik from Technion University in Tel Aviv, visited the group in August of this year.
Additionally, we develop novel strategies for constructing complex peptides, with a focus on cyclic and multi-disulfide architectures. These scaffolds provide enhanced stability and functionality, supporting peptide-based therapeutics that address challenges such as antimicrobial resistance and selective enzyme inhibition.
Beyond medicine, we explore the interface of peptide science and agriculture. We are developing peptide-based plant growth regulators that influence development, flowering, stress responses, and immunity in crops, as well as antimicrobial peptides that function as sustainable biopesticides against fungal, bacterial, and viral pathogens.
By integrating advances in protein and peptide chemistry with biomedical and agricultural applications, our group is building a versatile peptide and protein synthesis toolkit to strengthen both human health and global food security.

Our Research
Efficient Strategies for Peptide Cyclization
Cyclic peptides are emerging as powerful modulators of biomolecular interactions, particularly with targets that present large and complex binding surfaces. Compared with their linear counterparts, they offer improved stability, affinity, and cell permeability. Our group is developing efficient, user-friendly methods to generate structurally diverse cyclic scaffolds. These approaches expand the peptide engineering toolbox and accelerate the discovery of functional molecules for applications in chemical biology and drug discovery.
Peptide Drug Discovery
Leveraging our cyclization platform, we develop bioactive peptides with antibacterial and anticancer activity with high potency, protease resistance, and cell penetration. In parallel, we use mRNA display to screen large libraries of macrocycles for high-affinity binders to challenging, disease-relevant protein interfaces. To precisely control target identity, we apply chemical protein synthesis to produce site-specifically post-translationally modified proteins and their D-enantiomers, enabling selections against defined modification states and mirror-image targets. Together, these capabilities expand peptide-protein interaction mapping and accelerate the creation of drug-like cyclic peptides for chemical biology and therapeutics.
Chemical Protein Synthesis
Chemical protein synthesis offers a powerful route to obtain medium-sized proteins with high homogeneity in workable quantities, supporting a broad range of biochemical, structural, and functional studies. Using solid-phase peptide synthesis and chemoselective ligation, small to medium-sized proteins could be assembled efficiently. However, proteins prone to aggregation, hydrophobicity, or poor solubility remain challenging. To address these barriers, we are developing innovative strategies—such as removable solubilizing tags—to expand the range of proteins accessible by chemical synthesis.
Chemical Protein Modification
Selective chemical modification of proteins is a versatile tool in chemical biology, biotechnology, and drug discovery, enabling precise tuning of protein structure and function beyond natural biosynthesis. We are developing novel and site-specific modification strategies, with a particular focus on cysteine residues, whose low natural abundance makes them ideal handles for targeted conjugation. These approaches provide powerful means to engineer proteins with tailored properties for mechanistic studies and therapeutic applications.


Publications
-
Trifluoroacetic Acid Promotes Ultrafast Peptide Cyclization Mediated with Dimethylolurea. Org. Lett. 2025, 27 (31), 8597–8601. https://doi.org/10.1021/acs.orglett.5c02534.
-
The Peptide-Encoding CLE25 Gene Modulates Drought Response in Cotton. Agriculture 2025, 15 (11), 1–15. https://doi.org/10.3390/agriculture15111226.
-
Recent Advances in Antimicrobial Lipopeptide Fengycin Secreted by Bacillus: Structure, Biosynthesis, Antifungal Mechanisms, and Potential Application in Food Preservation. Food Chem. 2025, 489. https://doi.org/10.1016/j.foodchem.2025.144937.
-
Molecular Mechanism of Fusarium Fungus Inhibition by Phenazine-1-Carboxamide. J. Agric. Food Chem. 2024, 72 (27), 15176–15189. https://doi.org/10.1021/acs.jafc.4c03936.
-
Ribosomal Incorporation of Lithocholic Acid into Peptides for the De Novo Discovery Of Peptide-Lithocholic Acid Hybrid Macrocyclic Peptides. ACS Chem. Biol. 2024, 19 (7), 1440–1446. https://doi.org/10.1021/acschembio.4c00298.
-
Doxorubicin-Based ENO1 Targeted Drug Delivery Strategy Enhances Therapeutic Efficacy against Colorectal Cancer. Biochem. Pharmacol. 2024, 224, 116220. https://doi.org/10.1016/j.bcp.2024.116220.
-
Identification of Protein–Phenol Adducts in Meat Proteins: A Molecular Probe Technology Study. Foods 2023, 12 (23), 4225. https://doi.org/10.3390/foods12234225.
-
Elucidating the Multimodal Anticancer Mechanism of an Organometallic Terpyridine Platinum(II) N-Heterocyclic Carbene Complex against Triple-Negative Breast Cancer In Vitro and In Vivo. J. Med. Chem. 2023, 66 (6), 3995–4008. https://doi.org/10.1021/acs.jmedchem.2c01925.
-
Generation of Purple-Violet Chrysanthemums via Anthocyanin B-Ring Hydroxylation and Glucosylation Introduced from Osteospermum Hybrid F3’5’H and Clitoria Ternatea A3’5’GT. Ornam. Plant Res. 2021, 1, 1–9. https://doi.org/10.48130/OPR-2021-0004.
-
Chemical Approaches for the Preparation of Ubiquitinated Proteins via Natural Linkages. J. Pept. Sci. 2022, 28 (3), 1–15. https://doi.org/10.1002/psc.3367.
-
Examining the Role of Phosphorylation of P19INK4d in Its Stability and Ubiquitination Using Chemical Protein Synthesis. Chem. Sci. 2020, 11 (21), 5526–5531. https://doi.org/10.1039/c9sc06300e.
-
The Journey for the Total Chemical Synthesis of a 53 KDa Protein. Acc. Chem. Res. 2019, 52 (12), 3361–3371. https://doi.org/10.1021/acs.accounts.9b00372.
-
Diverse Fate of Ubiquitin Chain Moieties: The Proximal Is Degraded with the Target, and the Distal Protects the Proximal from Removal and Recycles. Proc. Natl. Acad. Sci. U. S. A. 2019, 116 (16), 7805–7812. https://doi.org/10.1073/pnas.1822148116.
-
Palladium-Mediated Direct Disulfide Bond Formation in Proteins Containing S-Acetamidomethyl-Cysteine under Aqueous Conditions. Angew. Chemie – Int. Ed. 2019, 58 (17), 5729–5733. https://doi.org/10.1002/anie.201900988.
-
Total Chemical Synthesis of Ester-Linked Ubiquitinated Proteins Unravels Their Behavior with Deubiquitinases. Chem. Sci. 2018, 9 (6), 1661–1665. https://doi.org/10.1039/c7sc04518b.
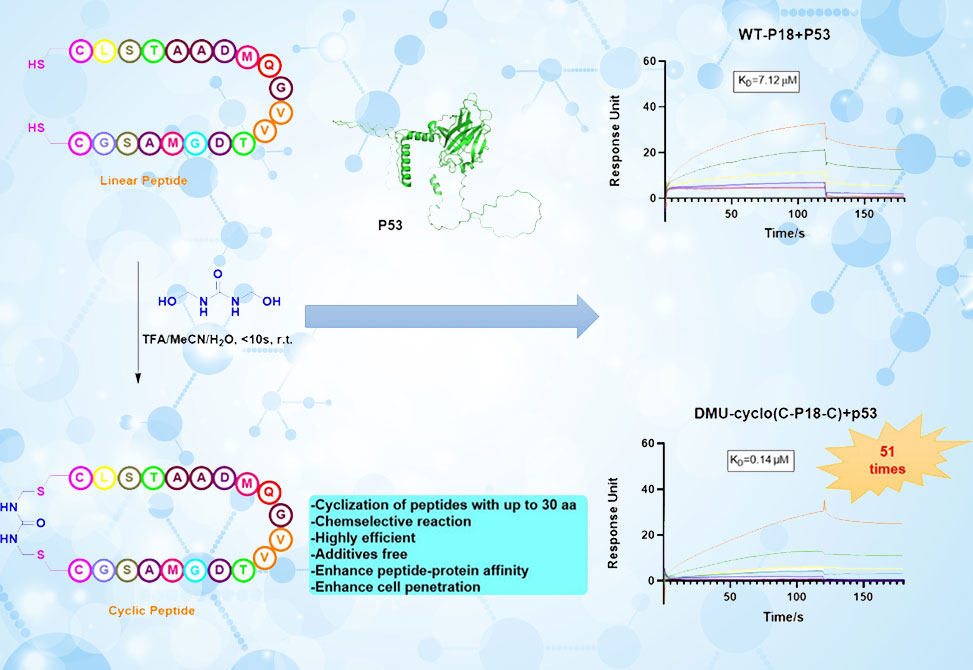
Ultrafast Peptide Cyclization
Read a condensed version of publication listed to the left as #1, highlighted on our website.
A Conversation with with Professor Hao Sun
College of Sciences, Nanjing Agricultural University
APS: Professor Sun, your recent work has attracted attention in both peptide chemistry and plant biology. Could you give our readers an overview of your current research directions?
Sun: My group works at the interface of synthetic chemistry and biology. On one side, we are exploring new methods for peptide modification and macrocyclization. For example, we recently reported how trifluoroacetic acid can promote ultrafast peptide cyclization mediated by dimethylolurea, a reaction that enables efficient stabilization of peptide structures. In parallel, we are interested in hybrid peptides—particularly ribosomal incorporation of lithocholic acid into peptides. This approach allows us to create peptide–lithocholic acid hybrids with novel macrocyclic topologies that are otherwise inaccessible by traditional synthetic strategies. On the biological side, we are pursuing peptide signaling in plants, with a recent focus on the CLE25 gene and its role in regulating drought responses in cotton. This dual focus reflects my broader interest in how peptide chemistry can be harnessed in both fundamental science and applied agricultural systems.
APS: Those are very different applications—fundamental peptide chemistry and plant stress physiology. How do you see these lines of work complementing one another?
Sun: At first glance, they appear separate, but they are connected by the versatility of peptides as signaling and structural molecules. Advances in synthetic peptide chemistry—such as the ability to build non-canonical scaffolds or cyclize peptides rapidly—give us a toolkit to probe biological questions. For instance, designing hybrid macrocycles expands the chemical space available for protein–protein interaction modulators. In plant systems, understanding the role of small peptides like CLE25 provides a foundation for engineering crops that can better tolerate environmental stresses. The unifying theme is that peptide science offers solutions across domains: from molecular probes that dissect biology to applications in agriculture where food security is directly affected.
APS: You mentioned your work on lithocholic acid–peptide hybrids. What motivated this line of research, and what potential do you see for such molecules?
Sun: Natural products like lithocholic acid have evolved to interact with biological membranes and proteins in ways that peptides alone cannot. By incorporating such hydrophobic moieties into ribosomally synthesized peptides, we can generate hybrid molecules with enhanced stability, permeability, and conformational control. These features are essential when attempting to modulate protein–protein interactions, which remain one of the most challenging classes of targets in drug discovery. Our early results show that peptide–lithocholic acid hybrids exhibit structural diversity and biophysical properties that justify deeper exploration. I see them as prototypes for a new class of macrocyclic modulators with potential in both medicine and crop science.
APS: The CLE25 study connects your work to agricultural applications. What are the implications of this research for plant science and food production?
Sun: Drought remains one of the greatest challenges to agriculture worldwide. The CLE25 peptide acts as a systemic signal, modulating stomatal closure and water use efficiency. By dissecting its role in cotton, we demonstrated how peptide-based signaling pathways contribute to stress adaptation. In the long term, understanding such pathways may allow us to design crops with enhanced resilience. While this is still fundamental research, it illustrates how peptide biology is not restricted to animal systems—plants also rely on peptides as critical regulators.
APS: Many of our readers are graduate students and postdocs. Could you share your perspective on mentoring the next generation of scientists?
Sun: I emphasize two principles: curiosity and cross-disciplinarity. The problems we face—whether in health or agriculture—rarely belong to a single field. Training young researchers to be fluent in both chemical methods and biological systems is essential. I encourage students to explore outside their comfort zones: chemists learning about plant genetics, biologists learning synthetic strategies. At the same time, mentorship means giving space for creativity while providing a rigorous foundation in experimental design. Watching students develop independence and generate ideas beyond what I could imagine myself is one of the most rewarding aspects of my career.
APS: Looking ahead, what directions do you hope to pursue in your laboratory?
Sun We are working to integrate our chemical and biological efforts more closely. On the chemistry side, we want to expand the repertoire of macrocyclic scaffolds and understand their conformational landscapes at high resolution. On the biology side, we are beginning to look at how synthetic peptides can be used to modulate plant signaling in controlled ways. The long-term vision is to create a feedback loop: chemistry informs biology, and biology motivates new chemistry. I believe this integration is where peptide science can have its most profound impact.

Professor Hao Sun
Professor Hao Sun was born in 1986. He completed his undergraduate studies, 2004–2008, in the Department for Intensive Instruction of Kuang Yaming Honors School, Nanjing University. He then obtained his Master’s and Ph.D., 2008–2013, at the School of Chemistry and Chemical Engineering, Nanjing University, under the supervision of Professor Yi Pan.
Following his doctorate, Sun worked as a research assistant at Nanjing University, 2013–2016, before undertaking postdoctoral research, 2016–2020, at the Technion–Israel Institute of Technology, where he trained under Nobel Laureate Aaron Ciechanover, 2004 Nobel Prize in Chemistry, and Professor Ashraf Brik, an APS 2025 Vincent du Vigneaud Award winner.
In 2020, Sun joined Nanjing Agricultural University as a Professor in the College of Sciences.
His research focuses on developing chemical methods for protein synthesis to produce highly homogeneous, high-purity modified proteins and other structurally complex proteins, enabling studies of their regulatory mechanisms in cells. This chemical protein synthesis technology overcomes the limitations of biological expression methods regarding purity and homogeneity, and allows for backbone design and side-chain modifications with important applications in chemical biology, synthetic biology, agriculture, biomedicine, and pharmaceuticals. He is also interested in developing peptide-based modulators for proteins related to diseases, with the goal of peptide drug discovery.
During his postdoctoral research, Sun successfully synthesized the largest chemically synthesized protein to date: tetra-ubiquitinated α-globin, comprising 472 amino acids and a molecular weight of 53 kDa. His groundbreaking work has been published in the Proceedings of the National Academy of Sciences, PNAS, garnering a commentary in that publication from his research peers, as well as in Accounts of Chemical Research, where it was featured as the cover article.
In 2024, at the 18th China International Peptide Symposium, 18th CPS, held in Hong Kong, Sun received the Young Peptide Scientist Award from the conference academic committee in recognition of his contributions to the peptide field.

