The demand for peptide therapeutics is booming, but their manufacture carries a heavy environmental burden. Solid-phase peptide synthesis, SPPS, the workhorse of the field, consumes vast quantities of hazardous solvents like N,N-dimethylformamide, DMF, and dichloromethane while generating substantial waste. Process mass intensity, PMI, the ratio of total materials to final product, remains stubbornly high. As peptide drugs proliferate, the demand for greener synthetic routes grows ever more urgent.
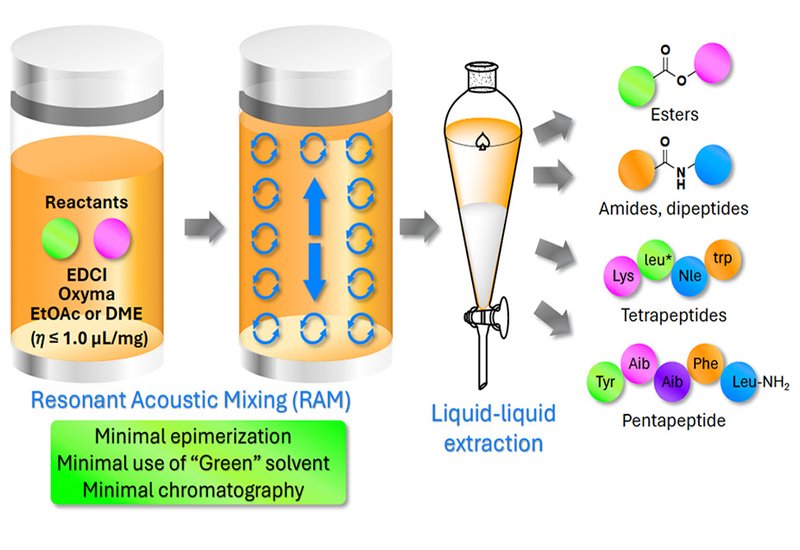
To address this challenge, Nassim Maarouf-Mesli and colleagues, led by Professors William D. Lubell and Felix Polyak at the Université de Montréal, reported in Green Chemistry an unconventional tool: resonant acoustic mixing (RAM). Unlike ball milling, which relies on mechanical grinding, RAM transfers energy through low-frequency acoustic vibrations (58–62 Hz) to generate uniform dispersion without crushing or increasing contamination risk. The team systematically evaluated RAM for high-concentration peptide bond formation using environmentally benign solvents, employing a water-soluble carbodiimide and Oxyma as coupling reagents to enable aqueous workup without chromatography.
Operating the RAM at 60–80 g0 proved critical. In green solvents such as ethyl acetate and 1,2-dimethoxyethane, DME, couplings reached completion within 10–15 minutes at concentrations as low as 0.9–1.0 μL mg−1. Liquid viscosity and base solubility emerged as key determinants of conversion efficiency; lower viscosity and lipophilic bases like Hünig's base yielded superior results. Comparative experiments confirmed that conventional stirring, sonication, and lower g0 agitation all failed to match the conversions achieved at 60 g0. Gram-scale synthesis of a model dipeptide in DME delivered 99% isolated yield after simple aqueous workup, demonstrating practical scalability.
Epimerization, the persistent nemesis of peptide synthesis, remained minimal across multiple challenging test systems. Phenylglycine couplings produced less than 3% of the undesired diastereomer, while valine-proline junctions, prone to oxazolone-mediated racemization, were optimized to under 7% epimerization by adjusting stoichiometry and omitting exogenous base. The notoriously difficult Aib-Aib coupling, joining two sterically hindered α-aminoisobutyric acid residues, proceeded in 82% yield using elevated reagent loading and extended mixing time. Process mass intensity before purification was three to five fold lower than conventional solution-phase methods and approximately two fold better after purification compared to both solution-phase and ball-milling approaches.
The team demonstrated broad substrate tolerance across ester, amide, and dipeptide synthesis. Dimethoxybenzyl and allyl esters were prepared with greater than 99.5:0.5 enantiomeric ratio. Fragment couplings of tetrapeptides relevant to interleukin-1 receptor antagonists and anti-amyloidogenic cyclic peptides proceeded successfully, confirming compatibility with alternating D- and L-configurations. As a culminating demonstration, Aib-enkephalin, a conformationally constrained opioid peptide benchmark, was assembled through sequential RAM-mediated couplings with aqueous workup replacing chromatography at each step.
This work positions resonant acoustic mixing as a practical platform for sustainable peptide manufacturing. By enabling rapid, high-concentration couplings in benign solvents with minimal waste, RAM addresses the field's growing environmental footprint. The approach proved compatible with standard Fmoc/t-Bu protecting group strategies while dramatically reducing solvent consumption. Future integration with supported liquid-phase or solid-phase platforms could extend RAM's utility to longer sequences. As peptide therapeutics continue their ascent, technologies that reconcile synthetic efficiency with environmental responsibility will prove essential, and resonant acoustic mixing offers a compelling path forward.