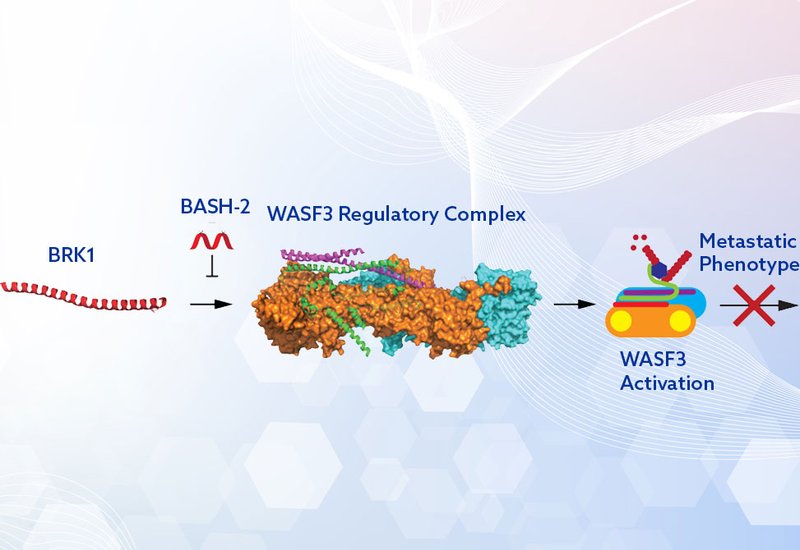
Metastasis remains the leading cause of cancer mortality, and one driver of this process is the actin cytoskeleton remodeling mediated by WASF3, a protein strongly upregulated in aggressive tumor types. Despite its centrality in invasion and metastasis, no effective probes have existed to directly disrupt WASF3 activity within its regulatory complex.
To address this challenge, researchers in the Eileen Kennedy Group at the university of North Carolina at Chapel Hill, report in ACS Medicinal Chemistry Letters the design of stapled peptide mimics of BRK1, a critical—but understudied—component of the WASF Regulatory Complex. Their approach leverages hydrocarbon-stapled helices to displace BRK1, destabilizing the WRC and downregulating WASF3 activity, thereby inhibiting cancer cell migration and invasion.
Using homology modeling of the WASF3–ABI2–BRK1 trimer and in silico alanine scanning, a hotspot within BRK1, residues 19–41, was identified as the interface mediating interaction with ABI2-WASF3. Two stapled peptides, BASH-1 and BASH-2, incorporating hydrocarbon staples at i,i+4 positions and solubilizing modifications, lysine substitutions, PEG3 linker, were synthesized. Circular dichroism confirmed enhanced α-helicity compared to the native sequence, and microscopy/flow cytometry demonstrated cellular uptake in MDA-MB-231 breast cancer cells.
Functionally, both BASH peptides reduced cell migration by 45–60% in wound-healing assays, with BASH-2 showing superior potency and clear dose dependence. Invasion assays across a Matrigel barrier confirmed BASH-2 as a robust inhibitor of invasion, achieving >90% reduction at 1 μM, outperforming a previously reported WASF3-derived stapled peptide, WAHMIS-2. Importantly, activity was sequence-specific, as scrambled controls were inactive, and treatment showed no cytotoxicity up to 48 h. Similar results were obtained in prostate cancer PC-3 cells, underscoring generalizability across WASF3-expressing cancers.
Mechanistically, biotinylated pulldown assays revealed BASH-2 interacts directly with ABI2 and WASF3, consistent with displacement of BRK1 and destabilization of the WRC. In contrast, WAHMIS-2 bound only ABI2, and scrambled controls lacked activity. Phalloidin staining demonstrated BASH-2 disrupted lamellipodia-associated actin localization at the leading edge, inducing stress fiber formation indicative of suppressed motility.
Collectively, this study introduces BASH-2 as the first BRK1-mimetic stapled peptide capable of permeating cells, engaging the WASF3 WRC, and potently inhibiting cancer cell migration and invasion. These results establish BRK1 as a druggable node within the WRC and validate stapled peptides as effective tools for targeting its helical protein–protein interfaces. The findings highlight potential for synergistic strategies, combining BRK1-derived inhibitors with other WRC-targeting peptides or upstream WASF3 regulators, for example, STAT3, PI3K, ABL inhibitors. By directly impairing metastatic processes, BASH-2 and related molecules may complement standard chemotherapies, reducing tumor burden and addressing the critical unmet need for anti-metastatic agents in aggressive cancers such as triple-negative breast cancer.