Immune cells naturally release extracellular vesicles studded with peptide-MHC complexes that can orchestrate immune responses, either directly stimulating T cells or transferring their cargo to antigen-presenting cells. Researchers have long sought to manufacture these antigen-presenting vesicles for cell-free immunotherapy, but existing approaches face persistent obstacles. Harvesting vesicles from patient-derived immune cells yields meager quantities. Microfluidic vesicles suffer short half-lives and rupture under the turbulent flow of the cardiovascular system. Polymer-coated nanoparticles conjugated with peptide-MHC complexes have shown therapeutic promise in autoimmune models, but their rigid spherical scaffolds limit the surface area available for T cell receptor engagement and prevent the lateral movement and clustering of MHC molecules that help form an effective immunological synapse.
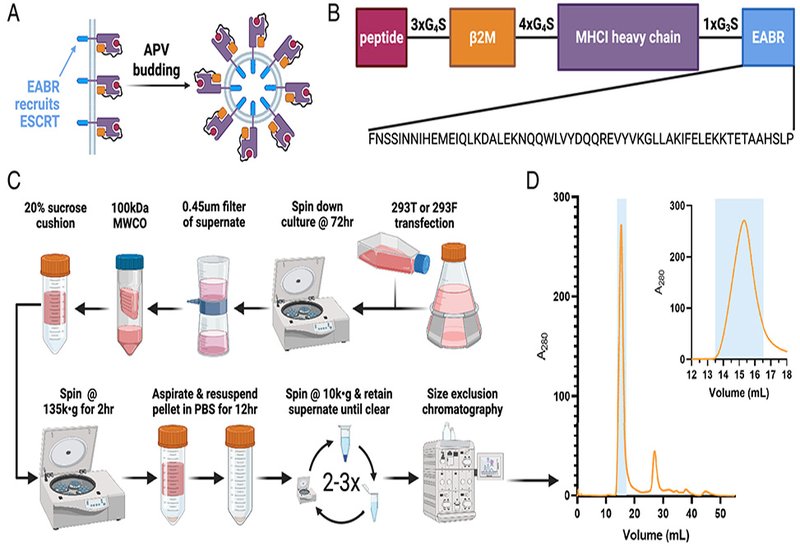
Researchers in the Mayo Group at Caltech, published in PNAS, engineered antigen-presenting vesicles that bud directly from nonimmune cells upon transfection with a single DNA or mRNA construct. The key innovation fuses an ESCRT- and ALIX-binding region from the human centrosomal protein CEP55 to the cytoplasmic tail of a single-chain heterotrimer peptide-MHCI. This EABR sequence recruits the cellular machinery normally responsible for membrane scission during cell division and viral budding. To ensure each vesicle displays the intended peptide rather than endogenous fragments, the team linked the presenting peptide to β2-microglobulin and the HLA-A*02:01 α chain through glycine-serine spacers. Removing either linker dramatically reduced the yield of correctly assembled complexes, confirming that the single-chain architecture is essential for loading the desired antigen.
ELISA measurements showed that appending the EABR sequence boosted vesicle production more than 100-fold compared to constructs lacking it. Immuno-electron microscopy revealed cup-shaped structures 60 to 80 nm in diameter, resembling canonical exosomes, with halos of gold-labeled anti-HLA antibodies confirming dense MHCI decoration. Mass spectrometry detected enrichment of both MHCI and the tetraspanin markers CD81 and CD63, further supporting an exosome-like identity. The platform generalized across human, murine, and chimeric MHCI variants, and mRNA transfection of murine mammary epithelial cells achieved up to 50-fold improvements in vesicle yield upon EABR addition. ELISPOT assays demonstrated that purified vesicles displaying the NY-ESO-1 cancer epitope stimulated IFN-γ release from T cells transduced with the cognate 1G4 receptor at levels comparable to conventional peptide-MHCI tetramers. Vesicles loaded with an irrelevant MART-1 peptide elicited no response above background, confirming antigen specificity.
Intriguingly, the vesicles induced substantially less IL-2 release than soluble tetramers or monomers, a profile that may favor immunosuppressive rather than immunostimulatory applications. Because IL-2 drives T cell proliferation and survival, dampened IL-2 alongside robust IFN-γ could redirect autoreactive T cells toward regulatory phenotypes without expanding them. The team tested constructs presenting NRP-V7 and IGRP epitopes relevant to type 1 diabetes, both of which have shown therapeutic potential when conjugated to iron-oxide nanoparticles in murine models. Unlike viral-vector or nanoparticle platforms, the EABR-mediated vesicles require no accessory coat proteins and derive entirely from sequences already present in the human proteome, potentially reducing immunogenicity. Because a single mRNA construct suffices for production, the approach could translate into injectable therapeutics that generate antigen-presenting vesicles directly in the patient, distributing throughout the body much as natural immunoregulatory vesicles do during an immune response.