Researchers in the Yamada Group at Tokyo University of Pharmacy and Life Sciences, published in Biochemistry, have systematically characterized LDV-derived peptides as cell-adhesive molecules targeting integrin α4β1. Their work identifies a minimal binding sequence, demonstrates the power of cyclization to enhance activity, and establishes these peptides as practical substrates for culturing immune cells.
Integrin α4β1 mediates immune cell adhesion and trafficking, guiding leukocytes from the bloodstream into inflamed tissues. It also contributes to the behavior of mesenchymal cells involved in tissue repair and regeneration. While the Arg-Gly-Asp motif has been extensively developed as a cell-adhesive ligand, its counterpart Leu-Asp-Val remains comparatively underexplored despite binding a distinct integrin subset with different biological functions. The team sought to fill this gap by identifying which LDV-related sequences best promote cell adhesion and determining how structural modifications might enhance their activity.
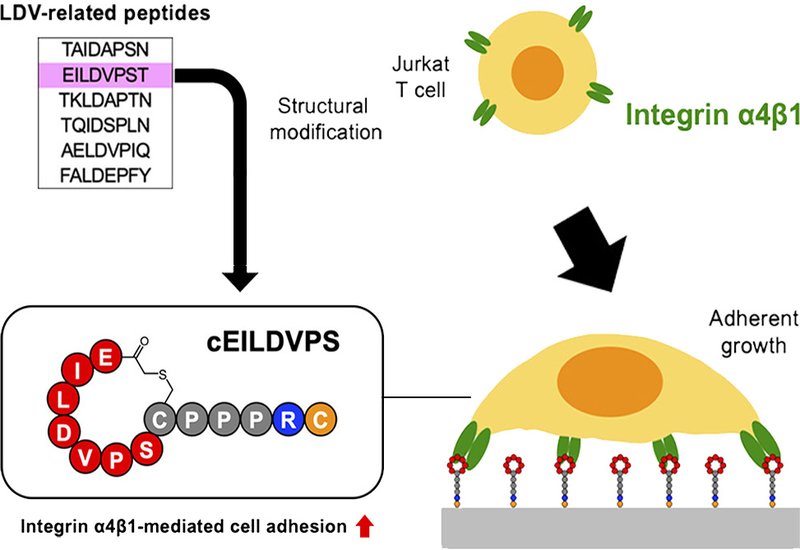
Group members synthesized six peptides containing LDV-related motifs drawn from human fibronectin, VCAM-1, and thrombospondin. Each sequence was immobilized on culture plates and tested for its ability to anchor Jurkat T cells, which express high levels of α4β1. Only one peptide, EILDVPST from the fibronectin CS1 domain, showed strong adhesion activity. The team then performed truncation and alanine-scanning analyses to map the essential residues. Removing the C-terminal threonine preserved full activity, establishing EILDVPS as the minimal active sequence. Substituting leucine or aspartate with alanine abolished binding almost completely, confirming these two residues as the critical core, while neighboring amino acids provided structural support.
The team then asked whether constraining the peptide conformation could boost performance. They cyclized EILDVPS by forming a thioether bond between the peptide termini. This modification dramatically increased adhesion activity compared to the linear form, providing direct experimental evidence that conformational constraint enhances α4β1 binding affinity. A control peptide with swapped LD residues showed no activity, confirming sequence specificity. The cyclic peptide cEILDVPS proved less potent than the high-affinity peptidomimetics BIO1211-C and LLP2A-C, which incorporate non-natural amino acid structures. However, all three ligands supported comparable long-term proliferation of Jurkat cells over seven days. Cells grown on these adhesive surfaces spread evenly rather than forming the aggregates typical of suspension culture, facilitating microscopy and improving experimental handling.
These findings position LDV-derived peptides as valuable tools for immune cell culture and biomaterial design. Because LDV targets α4 integrins rather than the αv integrins engaged by RGD, the two motifs address complementary cell populations and biological functions. Future work extending these studies to fibroblasts and mesenchymal stem cells could reveal how LDV peptides influence adhesion-dependent signaling in regenerative contexts. The study demonstrates that even modest peptide sequences, when rationally optimized through cyclization, can serve as effective cell-adhesive substrates for immunotherapy and tissue engineering applications.