Therapeutic peptides offer a powerful strategy for disrupting intracellular protein-protein interactions that small molecules cannot effectively target. However, delivering peptides to the cytosol presents a formidable challenge. Receptor-mediated endocytosis can internalize peptides into cells, but most cargo remains trapped within endosomal compartments rather than reaching the cytosol where therapeutic targets reside. Quantifying how much peptide actually escapes into the cytosol has proven equally difficult, creating a major roadblock for advancing this delivery strategy.
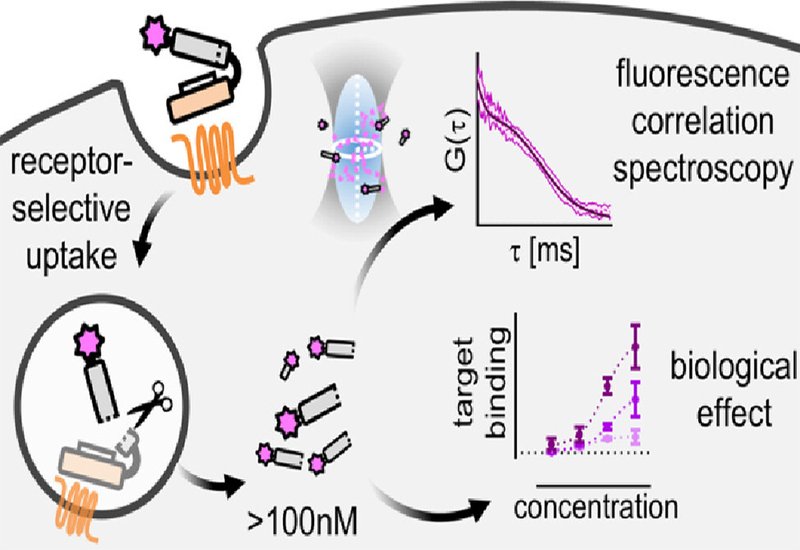
Researchers in the Beck-Sickinger Group at Leipzig University, published in JACS, developed a receptor-mediated shuttle system targeting the chemokine-like receptor 1, known as CMKLR1. This G protein-coupled receptor undergoes efficient internalization and shows overexpression in several cancer types including lung, prostate, and colorectal tumors. The team selected PMIγ as their model cargo, a D-amino acid peptide that binds MDM2 with nanomolar affinity. MDM2 regulates p53 levels and plays a key role in suppressing apoptosis across many cancers. The all-D configuration of PMIγ protects it from proteolytic degradation in the harsh endolysosomal environment, making it an ideal candidate for receptor-mediated delivery.
The researchers constructed modular peptide conjugates linking PMIγ to a cyclic variant of chemerin-9, a ligand that efficiently triggers CMKLR1 internalization. They incorporated a cathepsin-cleavable linker to release the cargo within endosomes and attached the far-red fluorophore sulfo-Cy5 for tracking. To enhance endosomal escape, they also prepared variants containing hsLMWP, a histidine-rich peptide that responds to the acidic pH of endosomes. Using fluorescence correlation spectroscopy, the team directly measured cytosolic peptide concentrations in living cells. This technique detects fluorescence fluctuations as molecules diffuse through the focal volume of a confocal microscope, providing absolute concentration values rather than indirect readouts. After 72 hours of incubation with 1 μM peptide, cells expressing CMKLR1 achieved median cytosolic concentrations of 44.6 nM for the basic conjugate and 98.8 nM with the hsLMWP enhancement. At 10 μM extracellular concentration, cytosolic levels reached 106 nM and 228 nM respectively. Many individual cells exceeded 100 nM, surpassing the 90 nM binding affinity of PMIγ for MDM2.
Bioluminescence resonance energy transfer experiments confirmed that the delivered peptides engaged their intracellular target. Cells expressing CMKLR1 showed strong BRET signals between NanoLuc-tagged MDM2 and the Cy5-labeled peptides, while cells lacking the receptor showed minimal signal. Competition with unlabeled chemerin-9 abolished receptor-specific uptake, confirming the delivery mechanism. Adding the MDM2 antagonist nutlin-3a displaced the peptides from MDM2, demonstrating specific binding. Unlabeled peptide conjugates successfully inhibited the p53-MDM2 interaction in a concentration-dependent manner, providing functional proof that receptor-mediated shuttling delivers biologically active cargo to cytosolic targets. These findings establish that receptor-mediated delivery can achieve therapeutically relevant intracellular concentrations without requiring membrane-disrupting cell-penetrating peptides, which often cause nonselective toxicity. The modular design allows straightforward optimization of both targeting ligand and therapeutic cargo, opening possibilities for selective delivery to receptor-expressing tumors.