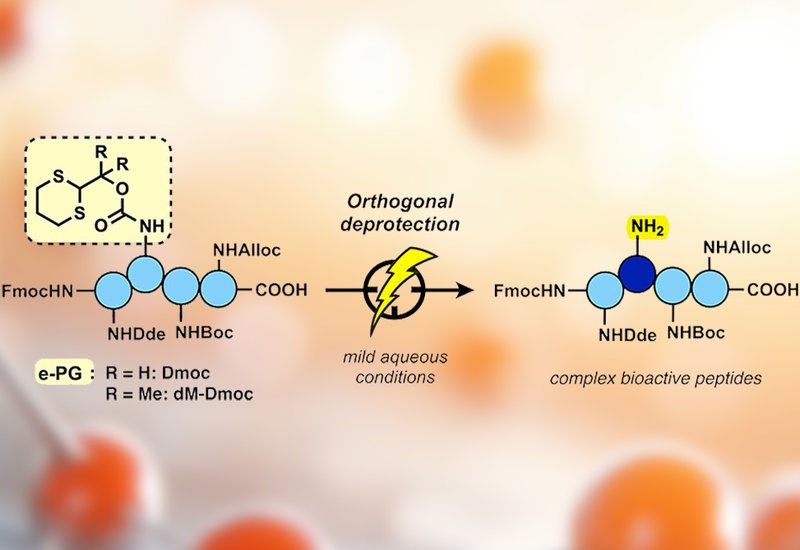
Protecting group chemistry sits at the heart of peptide synthesis. Every amide bond formed on a growing chain demands that other reactive side chains stay masked, and every mask must eventually come off cleanly without damaging the product. The standard Fmoc-based strategy handles this through layers of orthogonality: base-labile groups for the backbone, acid-labile groups for side chains, and palladium-cleavable or reductively labile groups for selective on-resin modifications. Yet each deprotection step typically consumes excess reagents, from piperidine and trifluoroacetic acid to odorous thiols and precious metal catalysts. As the peptide field pushes toward larger and more complex architectures, these chemical demands compound, challenging both sustainability and functional group tolerance. An entirely different trigger for protecting group removal, one that avoids stoichiometric reagents altogether, would open a genuinely new dimension in orthogonal peptide synthesis.
Researchers in the Malins Group at the Australian National University, published in Organic Letters, turned to electrochemistry as exactly that trigger. Building on earlier work showing that 1,3-dithiane esters can be cleaved by mild anodic oxidation, the team designed two dithiane-based carbamate protecting groups, Dmoc and dM-Dmoc, for the ε-amine of lysine. They synthesized Fmoc-compatible building blocks, confirmed their electroactivity by cyclic voltammetry, and optimized deprotection conditions using a benchtop ElectraSyn 2.0 potentiostat with a nickel electrode pair in a mixed acetonitrile/aqueous sodium acetate solvent system.
The critical test came with a model tetrapeptide carrying five different amine protecting groups simultaneously: Fmoc at the N-terminus and Alloc, Boc, Dde, and Dmoc or dM-Dmoc on the four lysine side chains. Systematic orthogonality studies confirmed that the Dmoc group survived palladium-mediated Alloc removal, hydroxylamine-based Dde cleavage, piperidine-driven Fmoc deprotection, and acid-catalyzed Boc removal, all in 79% to quantitative yield. After screening more than 100 electrochemical conditions, the team achieved selective Dmoc cleavage in 57–62% isolated yield using constant-potential electrolysis with alternating polarity on scales up to 90 mg. Mechanistically, anodic oxidation generates a dithiane radical cation that undergoes β-elimination to liberate the free amine, as supported by identification of 1,2-dithiolane 1-oxide by high-resolution mass spectrometry. The dimethyl-substituted variant dM-Dmoc proved unexpectedly acid-sensitive, undergoing removal in just 1% trifluoroacetic acid in acetonitrile, a finding that positions it as an alternative mild acid-labile protecting group. To probe broader applicability, the team applied Dmoc deprotection to two bioactive peptide scaffolds: a cysteine-containing heptapeptide known for hydrogel self-assembly, where the trityl thiol protection survived intact at 45–51% yield, and a DNA-binding SPKR repeat peptide bearing Pbf-protected arginine and tert-butyl-protected serine, giving 51–54% yield.
This work introduces electrochemistry as a practical new axis of orthogonality for peptide protecting group strategies. By replacing chemical reagents with an applied voltage, dithiane-based groups can be removed under conditions that leave every other common protecting group untouched. While yields and scalability will benefit from further optimization, particularly for future solid-phase implementations, the approach points toward a more sustainable paradigm for constructing complex peptide architectures in which the deprotection trigger is as mild and tunable as adjusting a dial.