Antibody conjugates represent a cornerstone of modern therapeutics, diagnostics, and imaging. By attaching payloads such as cytotoxins, fluorophores, or radionuclides to immunoglobulins, researchers can harness antibody specificity for targeted delivery. However, conventional conjugation methods that modify lysine or cysteine residues produce heterogeneous mixtures with variable drug loading, compromising potency, stability, and manufacturing consistency. Site-specific conjugation addresses these challenges by fixing both the location and number of attached molecules, but many current approaches require genetic engineering of the antibody itself.
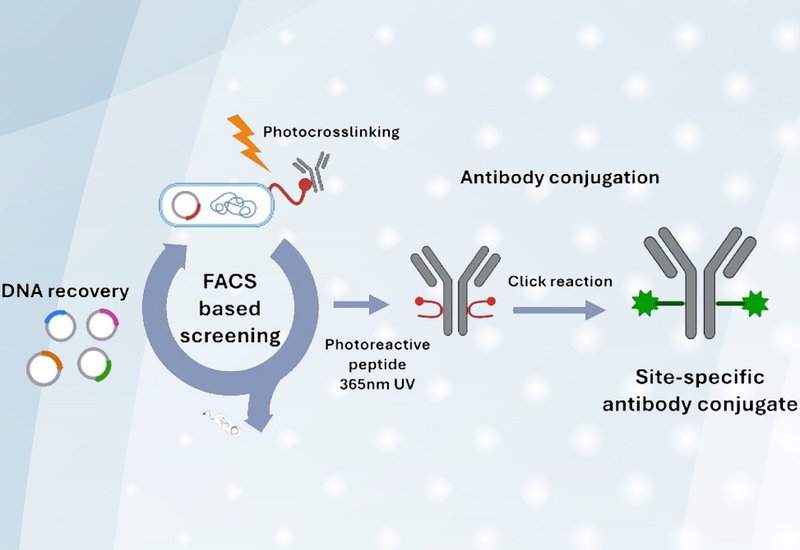
Researchers supervised by Professors Tae Hyeon Yoo and Eunha Kim at Ajou University, Korea, published in Bioconjugate Chemistry, report an evolved photo-cross-linking peptide that enables efficient site-specific modification of native antibodies without genetic engineering. The approach builds on their previously developed PEDIP method, which uses an Fc-binding peptide containing the photoreactive amino acid p-benzoyl-L-phenylalanine. Upon UV irradiation, the bound peptide forms a covalent bond with the antibody at a defined site. However, the original peptide required high concentrations and prolonged UV exposure, risking aggregation and photodamage.
To improve the system, the team developed a bacterial surface display platform that enables high-throughput screening of peptide libraries by fluorescence-activated cell sorting. They fused PEDIP peptide variants to a circularly permuted outer membrane protein on Escherichia coli, incubated the cells with human antibody, applied UV irradiation, and then used fluorescent secondary antibodies to detect covalently captured immunoglobulin. Cells displaying the most efficient cross-linking peptides were isolated by flow cytometry.
After screening two generations of libraries totaling millions of variants, a dominant clone designated B1 emerged with the sequence EPDCSYHLGELBWCTP, where B represents p-benzoyl-L-phenylalanine. The evolved peptide achieved 95.5% conjugation efficiency compared with 78.4% for the original sequence, and performed under milder conditions with shorter UV exposure and lower peptide concentrations. Mass spectrometry confirmed that B1 preserves the site specificity of the original peptide, forming its covalent bond exclusively at Met252 on the antibody heavy chain.
To enable downstream payload attachment, the researchers installed a cyclooctyne group at the N-terminus of B1. This modification proved critical for two reasons: it shifted the chromatographic retention of the conjugate for easier purification, and it provided a bioorthogonal handle for strain-promoted azide-alkyne cycloaddition chemistry. The cyclooctyne remained stable under the UV conditions required for photo-cross-linking, unlike other tested reactive groups such as dibenzocyclooctyne and tetrazines.
The team demonstrated the platform by conjugating trastuzumab with both a fluorescent dye and the cytotoxic payload monomethyl auristatin E. The resulting antibody-drug conjugate retained antigen specificity and exhibited potent cell killing in HER2-positive breast cancer cells with an IC50 of 119 picomolar, while showing minimal activity against HER2-negative cells. This directed evolution approach to engineering photo-cross-linking peptides opens opportunities for developing diverse antibody conjugates, including multispecific antibodies and antibody-oligonucleotide complexes, without modifying the antibody sequence.