Messenger RNA therapeutics have transformed medicine, from vaccines against infectious diseases to emerging applications in cancer immunotherapy and genome editing. Unlike DNA-based treatments that require nuclear entry, mRNA works directly in the cytoplasm to produce therapeutic proteins. However, delivering these large, negatively charged molecules into cells remains challenging. Current lipid nanoparticle platforms employ cationic or ionizable lipids that can trigger cytotoxicity and unwanted immune responses. Alternative peptide-based carriers offer better biocompatibility but have struggled to transport mRNA efficiently without transfection reagents.
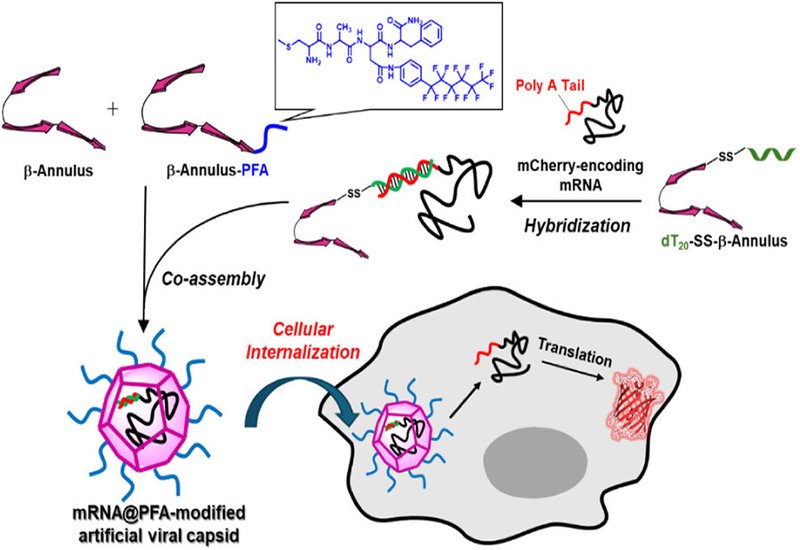
Researchers led by by Professor Kazunori Matsuura at Tottori University, published in Bioconjugate Chemistry, report an artificial viral capsid modified with perfluoroalkyl chains that delivers mRNA into cells safely and efficiently. The platform builds upon their previous work with the β-annulus peptide, a 24-residue sequence derived from tomato bushy stunt virus that spontaneously self-assembles in water to form hollow spherical structures resembling natural viral capsids.
The delivery system incorporates three peptide components. The first is the β-annulus peptide conjugated at its C-terminus with CADC6F13F, a cell-penetrating peptide bearing a perfluoroalkyl chain. The second is a β-annulus conjugate linked through a disulfide bond to a 20-nucleotide DNA strand that hybridizes with the poly-A tail of mRNA. The third component is unmodified β-annulus peptide, which improves capsid stability and reduces aggregation. When mixed with mRNA, these components self-assemble into spherical capsids approximately 200 nm in diameter that encapsulate the nucleic acid cargo.
The perfluoroalkyl modification proved critical for cellular uptake. Confocal microscopy revealed that fluorescently labeled capsids bearing 40% perfluoroalkylated peptide achieved 12-fold higher internalization into liver cancer cells compared with unmodified capsids. This optimal ratio balanced the membrane-penetrating benefits of the fluorinated chains against the aggregation tendency observed at higher concentrations. Mechanistic studies using endocytosis inhibitors indicated that the capsids enter cells primarily through caveolae-mediated pathways.
Cytotoxicity testing confirmed that 90% of cells remained viable after 24-hour incubation with the perfluoroalkyl-modified capsids, addressing a key limitation of cationic delivery systems. The capsids also showed preferential accumulation in cancer cells over normal kidney cells, suggesting potential for tumor-selective delivery.
Functional mRNA delivery was demonstrated using mCherry-encoding transcripts. Cells treated with the perfluoroalkyl-modified capsids showed 2.5-fold higher mCherry expression compared with lipofectamine-mediated delivery and 6-fold higher expression than capsids lacking the fluorinated modification. Colocalization experiments with lysosomal markers indicated successful endosomal escape, allowing the mRNA to reach the cytoplasm where translation occurs. The reducing environment of the cytosol cleaves the disulfide bonds linking mRNA to the peptide backbone, releasing the cargo for ribosomal processing.
This entirely peptide-based, noncationic delivery system addresses multiple challenges in mRNA therapeutics. The viral-mimetic architecture provides efficient encapsulation, the DNA hybridization strategy protects mRNA from degradation, and the perfluoroalkyl chains enable cellular uptake without the toxicity associated with conventional cationic carriers.