Two-dimensional materials have captivated researchers since graphene's isolation in 2004, offering properties unmatched by their bulk counterparts. High surface areas, tunable electronics, and morphological anisotropy make these ultrathin architectures promising for catalysis, sensing, and energy applications. Peptide-based approaches to 2D assembly hold particular appeal because they combine biocompatibility with programmable self-organization. Yet building robust nanosheets from peptide components remains challenging. Subtle changes in amino acid sequence often disrupt the delicate balance of forces driving lateral growth, and most peptide assemblies lack intrinsic fluorescence, requiring external probes for visualization.
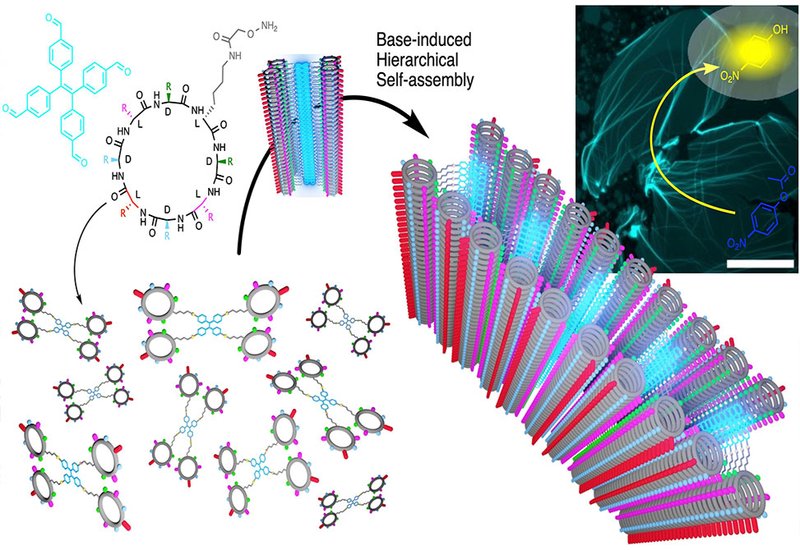
To address these limitations, researchers led by Professor Juan R. Granja at the University of Santiago de Compostela, collaborating with Javier Montenegro, also the corresponding author of the work, and Senior scientist Dr. Sebastien Ulrich at Institut des Biomolécules Max Mousseron, IBMM, at Montpellier, published in Angewandte Chemie International Edition, designed a hybrid scaffold that merges two powerful supramolecular motifs. They attached four nanotube-forming cyclic peptides to a central tetraphenylethylene, TPE, core through oxime linkages. The TPE component contributes aggregation-induced emission, meaning fluorescence switches on only when the molecules pack together, while the cyclic peptides drive pH-responsive stacking through β-sheet hydrogen bonding.
Alkalinization of acidic solutions triggered dramatic spectroscopic changes. Fluorescence emission at 495 nm increased sharply as pH rose above 6, coinciding with histidine deprotonation. Thioflavin T binding confirmed simultaneous formation of β-sheet structure, and wide-angle X-ray scattering revealed characteristic 4.2–4.7 Å spacings diagnostic of peptide nanotube assembly. Microscopy unveiled the true nature of these aggregates. Rather than forming bundled fibers as anticipated, the tetrameric building blocks spread into continuous fluorescent films spanning hundreds of square micrometers. Atomic force microscopy measured sheet heights of 3.7 nm, consistent with two stacked nanotubes flanking a tilted TPE core. Cryo-electron microscopy captured short nanotube intermediates of 20–70 nm, suggesting hierarchical growth in which initial tubular oligomers subsequently spread laterally.
The scaffold proved remarkably tolerant to structural variation. Substituting lysine with glutamine or glutamic acid shifted the pH stability window without disrupting 2D morphology. Repositioning histidine residues, replacing them with alanine or arginine, or methylating their imidazole nitrogens all yielded functional nanosheets. Modifications to the TPE core enabled precise thickness control, from 3.1 nm for a phenolic variant to 6.6 nm when polyethylene glycol chains occupied the hydrophobic interior. This tunability establishes the first molecular design strategy for controlling 2D supramolecular architecture height at the nanometer scale.
The aligned histidine residues decorating the nanosheet surfaces suggested catalytic potential. Indeed, assembled structures accelerated hydrolysis of p-nitrophenyl acetate more than eightfold compared to buffer alone. Adding zinc ions further enhanced activity, consistent with carbonic anhydrase-like metal coordination. Control peptides lacking the capacity for ordered assembly showed negligible catalysis, confirming that supramolecular organization of imidazole groups drives the enzymatic mimicry. These self-reporting, catalytically active nanosheets open new possibilities for biosensing platforms and artificial enzyme design, where fluorescence provides real-time feedback on assembly state while the ordered peptide surface performs useful chemistry.