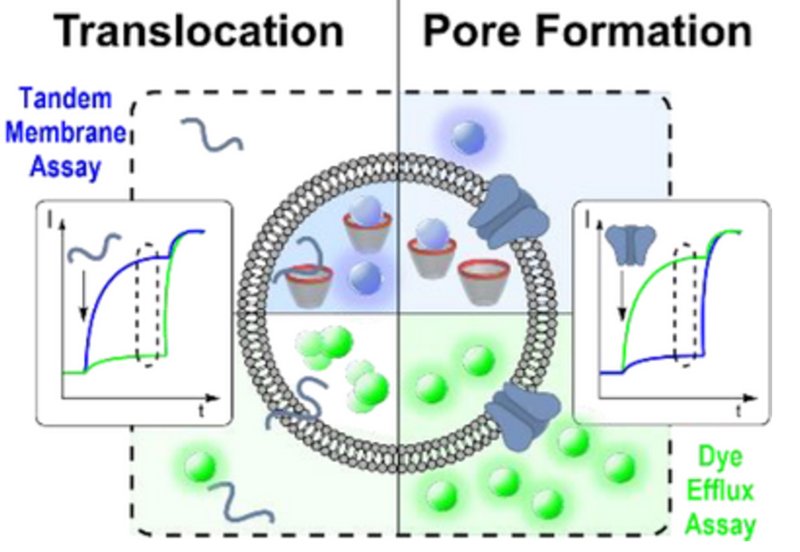
Cell-penetrating peptides and antimicrobial peptides both interact with lipid membranes, but with fundamentally different outcomes. Cell-penetrating peptides slip through membranes while leaving them largely intact, enabling cargo delivery without killing cells. Antimicrobial peptides punch holes that cause irreparable damage and cell death. Despite decades of research, distinguishing these behaviors experimentally has remained challenging because conventional dye efflux assays only detect pore formation, missing peptides that translocate silently without creating leaky membranes.
Scientists in Professor Werner Nau's group at Constructor University Bremen and Professor Andreas Hennig's laboratory at Universität Osnabrück have now developed a dual-channel fluorescence assay that simultaneously monitors both translocation and pore formation. Published in Angewandte Chemie International Edition, their approach combines a classical dye efflux assay using self-quenched carboxyfluorescein with their previously developed supramolecular tandem membrane assay, which encapsulates a host-dye reporter pair inside lipid vesicles. When peptides translocate across the membrane, they bind the encapsulated molecular host and displace the fluorescent dye, generating a signal even without pore formation.
The team optimized both assays to run under identical conditions, then developed spectral parameters allowing simultaneous monitoring in a single sample. Carboxyfluorescein excitation was shifted to 525 nm and emission monitored at 545 nm, while lucigenin was excited at 369 nm with emission at 475 nm, eliminating crosstalk between channels. Testing seven prototype membrane-active peptides revealed striking differences. Penetratin, Pep-1, and the minimal translocating peptide LRLLRW all showed significantly higher activity in the translocation channel than in the pore formation channel, statistically confirmed across seven independent vesicle preparations. These peptides cross membranes efficiently without creating holes large enough for dye escape.
The pore-forming peptide TP10 showed the opposite pattern: higher activity in the carboxyfluorescein channel, consistent with its known mechanism of creating membrane pores during translocation. Melittin, the hemolytic bee venom peptide, behaved similarly. The researchers then extended their method to distinguish transient from stable pores by adding spermine, a small molecule that can displace the reporter dye if it reaches the vesicle interior through open pores. After TP10 treatment, spermine addition produced no further signal, indicating the pores had closed. After melittin treatment, spermine caused continued signal increase, revealing stable equilibrium pores that remain open. This dual-channel platform offers a straightforward path toward high-throughput screening for peptide therapeutics, providing mechanistic classification that could accelerate development of both delivery vectors and antimicrobial agents.