Peptide therapeutics now account for roughly 8% of FDA-approved drugs, offering high target specificity and access to both intra- and extracellular targets that small molecules and biologics struggle to reach. Yet metabolic instability remains a fundamental vulnerability. Endogenous GLP-1 survives barely two minutes in the bloodstream before dipeptidyl peptidase-4 and neutral endopeptidase reduce it to fragments. More than 600 peptidase genes in the human genome ensure that proteolytic enzymes line virtually every tissue a peptide drug must traverse, from gastric lumen through hepatic first-pass metabolism into systemic circulation and beyond. Oxidative metabolism by cytochrome P450 enzymes, disulfide reduction, and phase II conjugation compound the challenge.
Researchers at Genentech, published in the Journal of Medicinal Chemistry, present a comprehensive perspective on the strategies that medicinal chemists and DMPK scientists now deploy to engineer metabolic resilience into therapeutic peptides. These strategies fall broadly into two categories. Direct structural modifications fortify the peptide itself: incorporating noncanonical amino acids such as D-isomers, N-methylated residues, α-alkylated variants like α-aminoisobutyric acid, or peptoid backbones to block peptidase recognition; constraining conformation through cyclization, disulfide bridges, or hydrocarbon stapling; and capping termini or fluorinating side chains to shield specific metabolic soft spots. Conjugation approaches increase the molecule's effective size and redirect clearance: lipidation promotes reversible albumin binding, PEGylation creates a steric shield against both peptidases and glomerular filtration, and fusion to albumin or immunoglobulin Fc fragments co-opts neonatal Fc receptor recycling to extend circulating half-life from minutes to days.
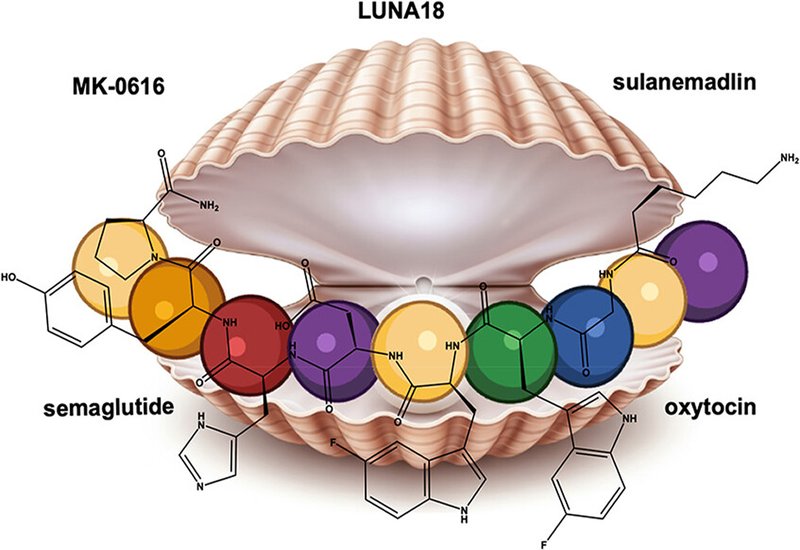
Five clinical-stage case studies illustrate these principles in action. Semaglutide pairs α-aminoisobutyric acid at position 8 for DPP-4 resistance with a C18 fatty diacid acylation at Lys26 for albumin binding, converting native GLP-1's two-minute half-life into 168 hours for once-weekly dosing. MK-0616, an oral tricyclic PCSK9 inhibitor discovered by mRNA display, overcame chymotrypsin, elastase, and trypsin liabilities through D-alanine incorporation, proline insertion, α-methylation, and a second macrocyclic ring closure that locked its binding conformation while blocking the remaining vulnerable amide bonds, achieving a Ki of 2.4 pM and 58% LDL cholesterol reduction in Phase II. LUNA18, designed as a cell-permeable cyclic peptide targeting the intracellular KRAS–SOS1 interaction, employed extensive backbone N-methylation and high lipophilicity to achieve oral bioavailability and entered Phase I as the first orally bioavailable peptide against an intracellular protein–protein interaction in oncology. Sulanemadlin leveraged a hydrocarbon staple to lock an α-helical conformation mimicking p53, with a polyalanine tail whose proteolytic cleavage actually generated a 10-fold more potent active metabolite. Oxytocin analogues illustrate the opposite extreme, a molecule so sensitive that even minor modifications can switch agonist to antagonist activity, demanding painstaking residue-by-residue optimization of disulfide mimetics, PEGylation, and side-chain substitutions to gain stability without sacrificing function.
Looking ahead, the authors envision AI and machine learning accelerating peptide design by predicting conformational ensembles, metabolic soft spots, and the chameleonic behavior that allows cyclic peptides to adapt their polarity between membrane and aqueous environments. As display technologies generate ever larger candidate libraries, systematic DMPK workflows integrating in silico predictions with tailored in vitro assays will be essential. The perspective makes clear that no single strategy suffices: the most successful peptide drugs layer multiple modifications to survive the metabolic gauntlet while retaining the potency and selectivity that make peptides a compelling therapeutic modality.