T-cell immunoglobulin and mucin domain 3, TIM-3, ranks among the most promising immune checkpoint targets in oncology. Expressed on exhausted T cells, natural killer cells, and tumor-associated macrophages, TIM-3 suppresses antitumor immunity by promoting tolerance within the tumor microenvironment. Noninvasive imaging of TIM-3 expression could guide patient selection for checkpoint blockade and monitor treatment response. Yet existing positron emission tomography, PET, tracers fall short: antibody-based probes circulate for days before clearing, while L-configured peptide tracers succumb rapidly to proteolytic degradation in vivo.
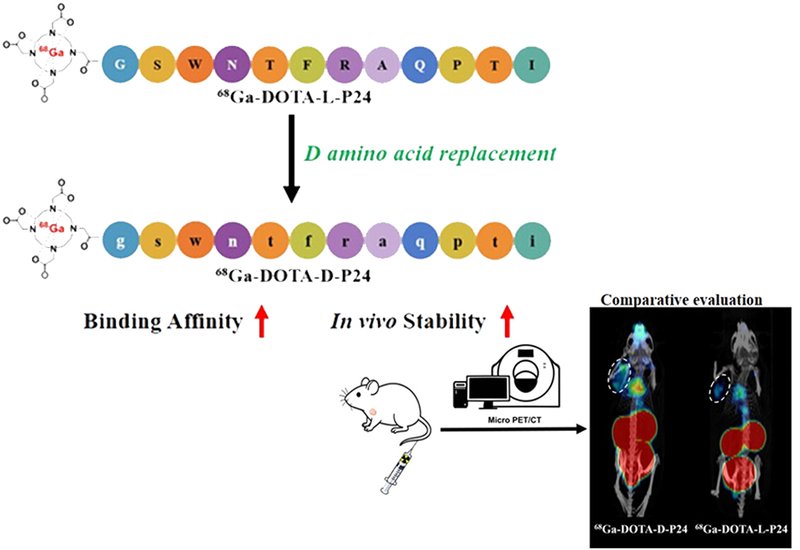
A team led by Zonghua Luo at Shanghai Tech University, Shanghai, China, published in Bioconjugate Chemistry, reasoned that replacing every amino acid in a TIM-3-targeting peptide with its mirror-image D-enantiomer would confer protease resistance without sacrificing binding. Starting from P24, a 12-residue sequence identified through phage display, they conjugated the chelator DOTA to the N-terminus and synthesized both L- and D-configured versions using standard Fmoc solid-phase chemistry. AlphaFold3 molecular docking predicted that the D-peptide would adopt a different binding pose within the same TIM-3 pocket, potentially improving affinity. Radiolabeling with gallium-68 proceeded efficiently, yielding tracers with greater than 98% radiochemical purity.

Members in the Luo Group at ShanghaiTech University are dedicated to the strategic optimization of radiolabeled peptide-based ligands, bridging molecular discovery with applications in diagnostic imaging, PET, and targeted radionuclide-therapy.
The mirror-image switch delivered striking benefits. Biolayer interferometry revealed that DOTA-D-P24 bound TIM-3 with a dissociation constant of 0.43 μM, a 36-fold improvement over the L-peptide's 15.4 μM. Circular dichroism spectra confirmed the expected mirror-image relationship between enantiomers, ruling out racemization during synthesis. Stability tests proved equally dramatic. In mouse serum at 37 °C, the D-tracer retained 95% structural integrity after 90 minutes while its L-counterpart dropped to 77%. The gap widened further in vivo: 30 minutes after injection into mice, 89% of 68Ga-DOTA-D-P24 remained intact in plasma compared with just 8% of the L-version. Both tracers cleared rapidly through the kidneys, yielding clean background signal.
PET/CT imaging across six xenograft models, including gastric, pancreatic, colon, breast, and two lung cancer lines, revealed heterogeneous uptake patterns reflecting varying TIM-3 expression levels. MGC-803 gastric carcinoma offered the best combination of tumor uptake and background contrast. Head-to-head comparison confirmed that the D-tracer achieved 1.6-fold higher tumor accumulation than its L-enantiomer, and competitive blocking with unlabeled peptide reduced uptake by 31%, confirming TIM-3-specific binding.
The tracer also proved sensitive to immunological changes. When the researchers stimulated tumors with interleukin-15, a cytokine known to activate T cells and upregulate TIM-3 expression, PET imaging detected a 37.8% increase in tumor signal over baseline. Western blotting confirmed that TIM-3 protein levels had indeed risen. This demonstration suggests 68Ga-DOTA-D-P24 could serve as a pharmacodynamic biomarker, tracking how tumors respond to immunomodulatory therapies in real time. With no TIM-3 PET tracers yet approved for clinical use and several anti-TIM-3 antibodies advancing through clinical trials, this D-amino acid strategy offers a compelling path toward noninvasive immune monitoring in checkpoint-targeted oncology.