Cell-penetrating peptides, CPPs, can cross the plasma membrane and deliver diverse cargo, but directing them to specific organelles such as mitochondria remains difficult. The inner mitochondrial membrane is a particularly challenging barrier due to its dense protein composition and strong negative electrochemical potential. Mitochondria-targeting CPPs have been explored using alternating hydrophobic and cationic residues, yet achieving selective and efficient uptake is still limited.
In work published in ACS Chemical Biology, Adeline Schmitt, under the guidance of Helma Wennemers at ETH Zürich, investigated rigid polyproline II, PPII, helical scaffolds containing guanidinium-proline, Gup, residues patterned with hydrophobic groups to tune amphipathicity and uptake. They synthesized amphipathic nonapeptides of general sequence (XZZ)3, Z = Gup, where X was valine, phenylalanine, tryptophan, or cyclohexylalanine, Cha. Circular dichroism confirmed stable PPII helices with aligned hydrophobic and cationic edges. Cellular uptake in MCF-7 cells revealed that only the Cha-containing peptide achieved mitochondrial localization, while others remained cytosolic or nucleolar, whereas Arg-substituted controls lacking helicity failed to reach mitochondria, underscoring the importance of both secondary structure and hydrophobicity.
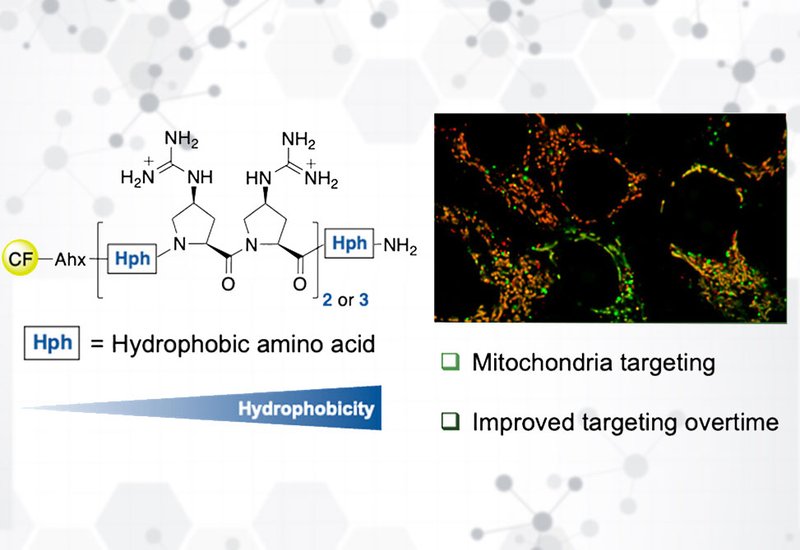
Hydrophobicity tuning further refined targeting. Adding a Cha residue at the C-terminus significantly increased uptake and mitochondrial colocalization, even in shorter 7-mer analogs. Introducing (4S)-cyclohexyl-proline, ChPro, directly into the backbone produced even more hydrophobic peptides. These variants showed higher uptake and faster membrane association, with some transitioning into mitochondria after 24 h; however, excessive hydrophobicity led to endosomal trapping, strong membrane binding, and cytotoxicity at micromolar levels.
Flow cytometry quantified these effects, showing up to 10-fold improvements in uptake upon Cha or ChPro substitution, while confocal microscopy clarified that high uptake often reflected membrane accumulation rather than true mitochondrial entry. Time-course studies revealed that several ChPro-containing peptides gradually redistributed from membranes and endosomes into mitochondria over 24 h, with stable intracellular persistence confirmed by lysate stability assays.
This work defines design rules for mitochondria-targeting CPPs: a rigid PPII backbone with guanidinium groups aligned on two edges and hydrophobic residues on the third edge enables selective uptake. Subtle increases in hydrophobicity—particularly at the C-terminus—enhance mitochondrial localization, though excessive hydrophobicity promotes off-target accumulation and toxicity. Importantly, intracellular redistribution over time proves critical, suggesting that both sequence design and temporal dynamics determine organelle selectivity.