Published in Angewandte Chemie International Editition, researchers from the Hackenberger Lab at the Humboldt-Universität in Berlin, present a powerful new class of phosphine oxide–based reagents for chemoselective protein modification, advancing both antibody–drug conjugate, ADC, design and peptide–protein conjugation strategies. The central innovation is the development of unsaturated phosphine oxides that can be modularly derivatized via CuI-catalyzed azide–alkyne cycloaddition, CuAAC, producing diethynyl-triazolyl-phosphine oxides, DTPOs, and ethynyl-ditriazolyl-phosphine oxides, EDPOs, with distinct reactivities and applications.
DTPOs act as highly efficient reagents for antibody disulfide rebridging, enabling homogeneous conjugates with rapid kinetics and superior stability. When applied to trastuzumab, only five equivalents of DTPO were sufficient to achieve complete rebridging within two hours, outperforming earlier diethynyl-phosphinate reagents that required overnight reactions. The resulting conjugates retained antigen selectivity and serum stability, and functionalization with tetrazine handles permitted modular payload attachment through inverse electron-demand Diels–Alder, IEDDA, chemistry. This two-step route produced a DAR ~4 trastuzumab–MMAE ADC with potent cytotoxicity against Her2-positive cells while sparing antigen-negative lines. The use of tetrazine/TCO intermediates ensures versatility across payload classes and minimizes direct handling of cytotoxic species.
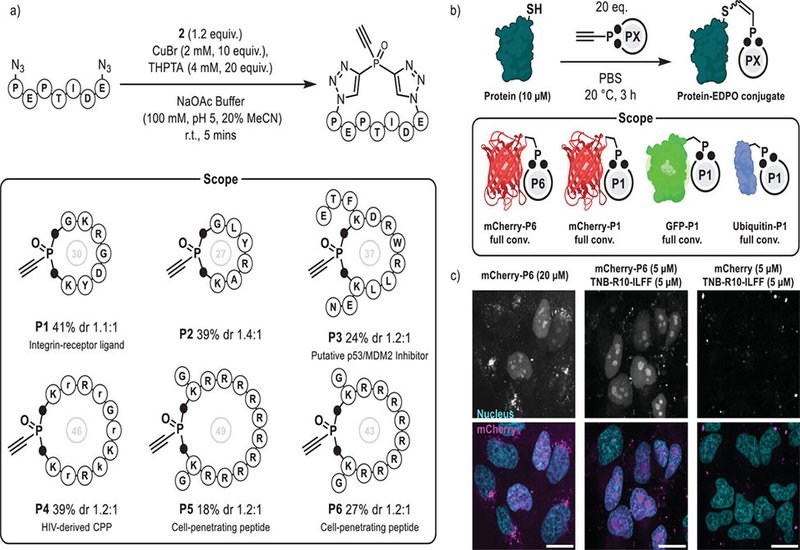
Parallel to antibody modification, EDPOs emerged from double CuAAC functionalization of triethynyl-phosphine oxide. These reagents uniquely combine peptide cyclization with installation of a cysteine-reactive handle, allowing one-pot peptide macrocyclization and subsequent protein conjugation. Using azide-functionalized peptides, the authors synthesized cyclic electrophilic ligands—such as integrin-binding RGD motifs and polyarginine-based cell-penetrating peptides—that were then conjugated to proteins like mCherry. The resulting peptide–protein hybrids displayed efficient intracellular delivery, especially when combined with cell-penetrating peptide additives, underscoring the utility of this chemistry for live-cell applications.
Overall, the work establishes unsaturated phosphine oxides as versatile triple-reactive scaffolds for bioconjugation. DTPOs provide fast, selective, and stable antibody rebridging, supporting modular ADC construction, while EDPOs introduce a unique single-reagent cyclization–bioconjugation platform for peptide–protein conjugates. These advances significantly expand the chemical biology toolkit, offering modular and orthogonal approaches for precise biomolecule functionalization. By bridging metabolic logic, synthetic efficiency, and biological function, this chemistry paves the way for next-generation ADCs, stable cyclic peptides, and novel therapeutic conjugates.