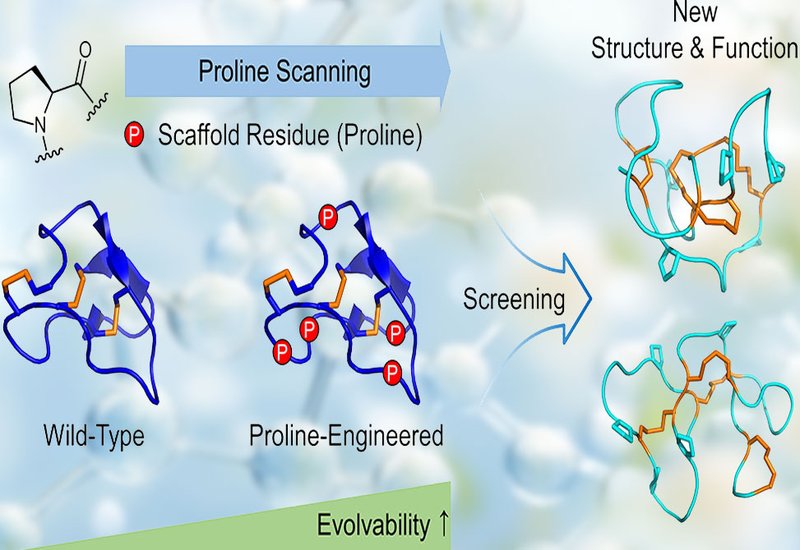
Disulfide-rich peptides, DRPs, are renowned for their stability and therapeutic promise, yet their natural structures offer limited flexibility for evolution or functional adaptation. In an innovative and collaborative study, researchers from the Wu group at the Xiamen University, Yin group at the Shandong University's Institute of Microbial Technology, and Tsai group at the Institute of Molecular Physiology, Shenzhen Bay Laboratory, published in J.A.C.S., present a proline-scanning strategy that enhances the evolvability of DRP scaffolds by promoting structural separation between rigid "scaffold" residues and mutable "nonscaffold" regions. The result is a new class of highly stable, adaptable cyclic peptides capable of binding challenging targets with precision.
Inspired by the unique conformational rigidity of proline, the team introduced up to seven additional proline residues into the inhibitor cystine knot, ICK, fold. These substitutions increased peptide folding efficiency dramatically, exceeding 90% in optimized variants, while maintaining structural integrity. Nuclear magnetic resonance, NMR, confirmed that even heavily proline-engineered DRPs preserved core topologies and introduced new scaffold polarity, enabling extensive sequence diversification without compromising foldability.
By integrating mRNA and phage display, researchers screened libraries of proline-engineered ICKs against key targets including TROP2, a tumor-associated antigen, and 4-1BB, a T-cell co-stimulatory receptor. The peptides identified showed high oxidative folding yields and low-nanomolar binding affinities, as low as 7.0 nM. Structural studies revealed novel disulfide pairing topologies and 3D folds, underscoring the capacity of these engineered DRPs to adopt entirely new architectures and functions.
The team demonstrated the therapeutic potential of DRPs by incorporating the TROP2-binding peptide pCT2 into a chimeric antigen receptor, CAR, construct. Compared to traditional scFv-based CARs, pCT2-CAR T cells selectively killed TROP2-overexpressing cancer cells while sparing cells with low antigen expression—greatly reducing off-tumor toxicity. These DRP-based CARs also secreted lower levels of pro-inflammatory cytokines, suggesting a safer profile with diminished risk of cytokine release syndrome.
This study introduces proline scanning as a powerful tool for engineering DRPs with enhanced structural polarity, folding efficiency, and functional diversity. By resolving the long-standing tradeoff between peptide rigidity and flexibility, the approach opens the door to next-generation cyclic peptides for drug discovery, immunotherapy, and beyond. The fusion of rational scaffold design with high-throughput screening platforms represents a major leap forward in modular, evolvable peptide therapeutics.