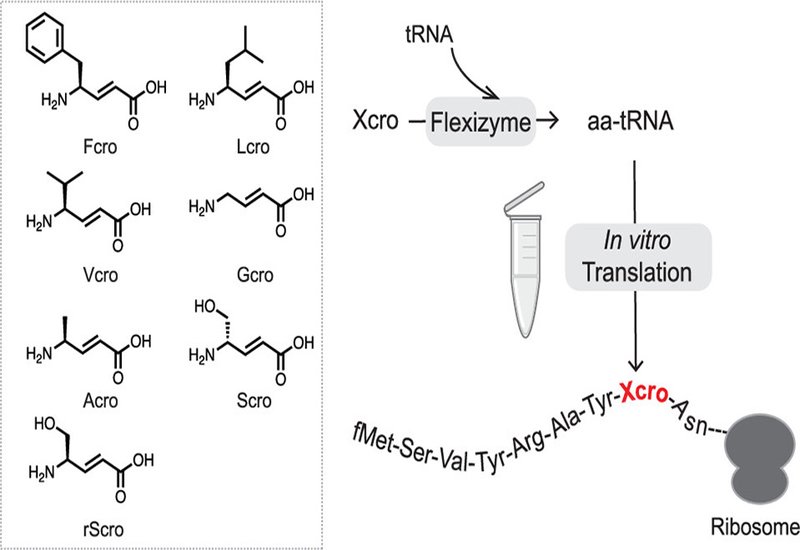
Trans-4-aminocrotonates, or Xcro, are α,β-unsaturated γ-amino acids found in bioactive marine natural products such as thalassospiramide and cyclotheonamide. Their rigid trans-alkene backbone confers proteolytic resistance and structural preorganization, making them attractive building blocks for drug-like peptides. Yet harnessing these residues in diverse peptide libraries has proven difficult: the biosynthetic enzymes that install Xcro tolerate few sequences, while conventional γ-amino acids stall ribosomal translation through rapid lactamization and poor fit within the peptidyl transferase center.
Researchers in the Hiroaki Suga Group at the University of Tokyo hypothesize in the Journal of the American Chemical Society, that the conformational constraint of Xcro's double bond might sidestep the lactamization problem, leaving ribosomal accommodation as the primary bottleneck. To test this, they synthesized a panel of Xcro derivatives bearing side chains mimicking canonical residues, including phenylalanine, Fcro, leucine, Lcro, valine, Vcro, and serine, Scro. Using flexizyme-catalyzed aminoacylation, they charged an engineered tRNA scaffold, tRNAPro1E2, with each variant and introduced them into a custom flexible in vitro translation, FIT, system.
Initial trials revealed truncated products, signaling that peptidyl transfer was rate-limiting. Systematic titration of elongation factors EF-Tu/Ts, EF-G, and EF-P identified conditions that nearly eliminated drop-off and boosted full-length yields roughly two-fold across all Xcro variants tested. Codon reassignment proved flexible: CCG, CAC, and UAC codons each accepted Fcro or Lcro without template-specific penalties. Dual incorporation succeeded when γ-residues were spaced by three or four intervening amino acids, though consecutive insertions stalled elongation. Crucially, Xcro-containing peptides underwent efficient thioether macrocyclization when paired with N-terminal chloroacetyl initiators and internal cysteines, yielding cyclic scaffolds compatible with display-based screening.
Beyond backbone integration, Xcro offers a handle for late-stage modification. Conjugate addition with thiols and phosphines proceeded with moderate stereoselectivity, and intramolecular cyclization via cysteine attack on an N-terminal Gcro residue generated macrocycles spontaneously at mild pH. Compared with dehydroalanine, Xcro displays intermediate electrophilicity, balancing reactivity with ribosomal compatibility.
By enabling direct ribosomal incorporation of conformationally constrained γ-amino acids, this work expands the chemical alphabet available to mRNA-displayed peptide libraries. The approach requires no ribosome engineering and integrates seamlessly with established RaPID selection workflows, opening a path toward structurally novel macrocyclic ligands for therapeutic targets.