Peptide Publications Archive
Showing items in Synthesis & Methodology · Clear filter
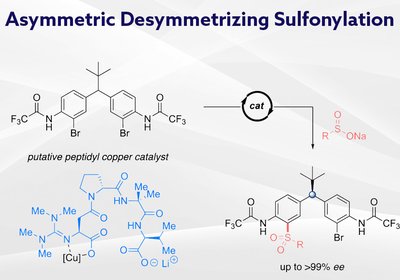
Remote Stereocontrol
Miller Lab
Researchers in the Miller Group at Yale University, published in the Journal of the American Chemical Society, have developed guanidinylated peptide ligands that enable …
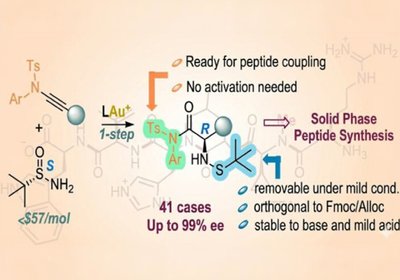
Ready-Made Amino Acids
Liming Zhang Lab
Researchers in the Zhang Group at the University of California, Santa Barbara, published in the Journal of the American Chemical Society, developed a concise …

Green Peptide Synthesis
Wellings, Meldal, and Wade Groups
Researchers supervised by Dr. Donald A. Wellings, Nobel Laureate Professor Morten Meldal, and Professor John D. Wade at the University of Melbourne, published in Nature …
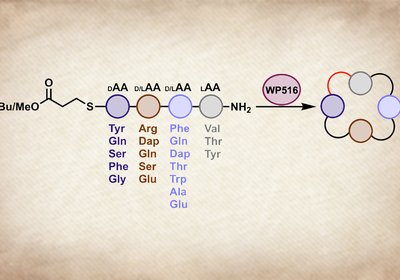
Tetrapeptide Cyclase
Parkinson Lab
Cyclic tetrapeptides occupy a tantalizing corner of chemical space. Head-to-tail macrocyclization eliminates the charged termini that limit membrane permeability, while the constrained
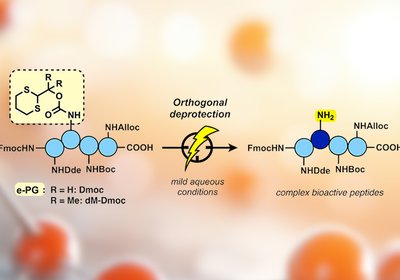
Electrochemical Deprotection
Malins Lab
Protecting group chemistry sits at the heart of peptide synthesis. Every amide bond formed on a growing chain demands that other reactive side chains stay …
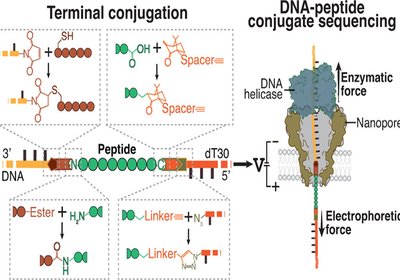
Threading Peptide Sequences
Dekker & Albada Labs
Researchers in the Dekker Group at Delft University of Technology, in collaboration with Bauke Albada at Wageningen University, published in the Journal of the American …
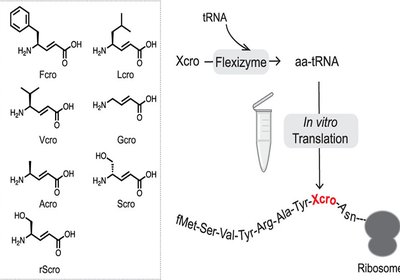
Ribosomal γ-Amino Acids
Suga Lab
Researchers in the Hiroaki Suga Group at the University of Tokyo hypothesize in the Journal of the American Chemical Society, that the conformational constraint …
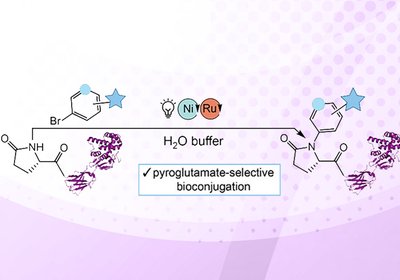
Pyroglutamate Labeling
Ball Lab
Scientists in the laboratories of Professors Zachary Ball and Laura Segatori at Rice University have developed a photoredox catalysis method that enables site-selective modification of …
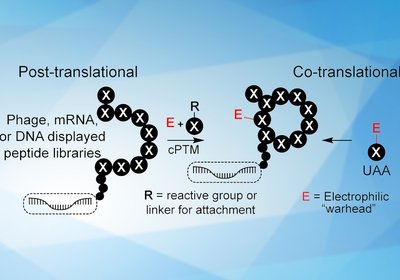
Covalent Library Design
Derda Group
Researchers in the Derda Group at the University of Alberta, published in Biochemistry, have surveyed the emerging field of covalent genetically-encoded libraries for discovering …
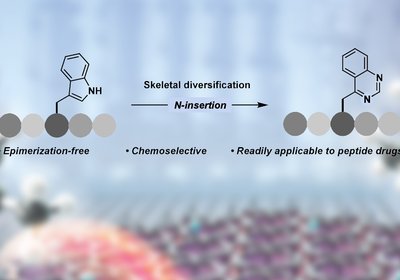
Tryptophan Transformation
Grob and Morandi Labs
A collaboration between the laboratories of Professor Bill Morandi in the Laboratorium für Organische Chemie and Dr. Nathalie Grob in the Institute of Pharmaceutical Sciences, …
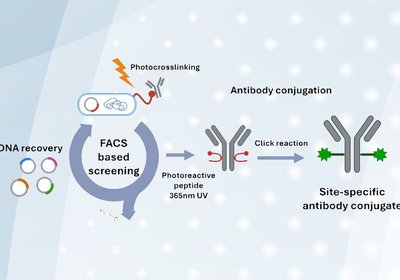
Evolved Photo-Cross-Linkers
Yoo & Kim Labs
Antibody conjugates represent a cornerstone of modern therapeutics, diagnostics, and imaging. By attaching payloads such as cytotoxins, fluorophores, or radionuclides to immunoglobulins, researchers can harness …
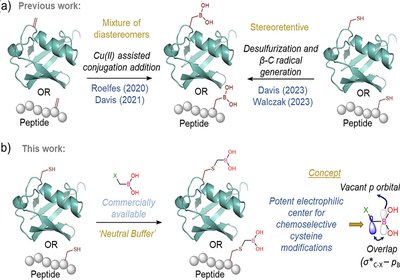
Cysteine Boronation
Bandyopadhyay Lab
Boronic acids have earned a versatile reputation in chemical biology and drug design. Their empty p orbital allows dynamic switching between sp2 and sp …
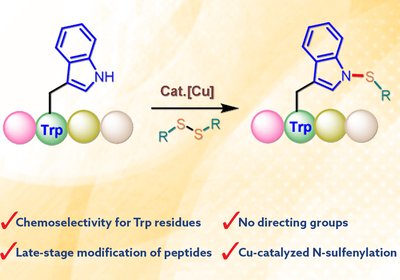
Tryptophan N-Sulfenylation
Yuan Lab
Peptides have become increasingly important as drug candidates and research tools due to their favorable safety profiles and ability to modulate protein-protein interactions. However, native …
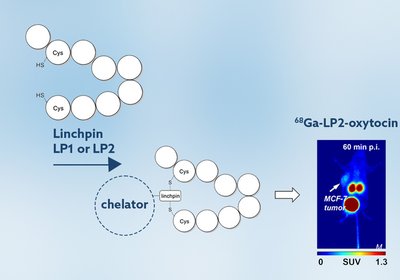
Oxytocin Receptor Imaging
Wuest Lab
Breast cancer remains a leading cause of cancer-related death worldwide, with tumor heterogeneity and absent biomarkers limiting diagnostic and therapeutic options. While PET tracers targeting …

Chiral Prodrug Synthesis
Kong Lab
Carboxylic acid groups frequently serve as essential pharmacophores in drug molecules, yet their high polarity limits membrane permeability and can reduce bioavailability. Prodrug strategies address …
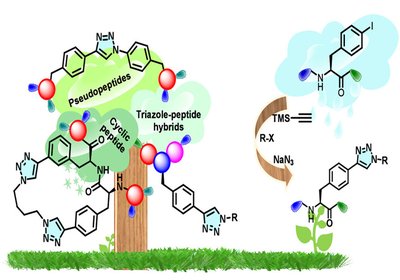
Clicking Safely
Jain Lab
The 1,2,3-triazole ring has become indispensable in peptidomimetic design. Its geometry and electronic properties closely mimic the trans-amide bond: similar planarity, comparable dipole moment, and …
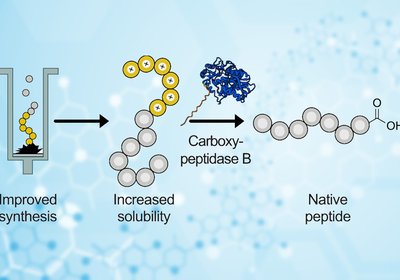
Taming Aggregation
Hartrampf Lab
Peptide chains misbehave. During solid-phase peptide synthesis, growing chains can collapse into sticky β-sheet structures that clog the resin, block coupling reactions, and leave chemists …
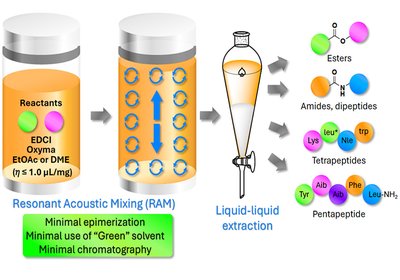
Acoustic Coupling
Lubell Group
The demand for peptide therapeutics is booming, but their manufacture carries a heavy environmental burden. Solid-phase peptide synthesis, SPPS, the workhorse of the field, consumes …
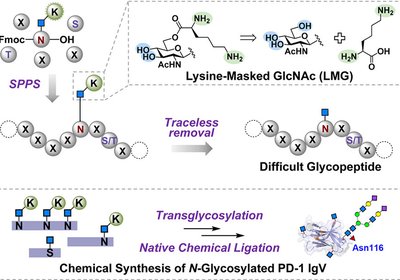
Masked Sugars
Dong Lab
Chemical synthesis provides atomic-level control over glycoprotein structure, yet hydrophobic sequences often aggregate before chemists can purify or ligate them. This problem may intensify for …
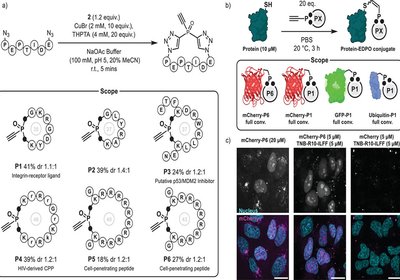
Phosphine Rebridging
Hackenberger Lab
Published in Angewandte Chemie International Editition, researchers from the Hackenberger Lab at the Humboldt-Universität in Berlin, present a powerful new class of phosphine oxide–based …
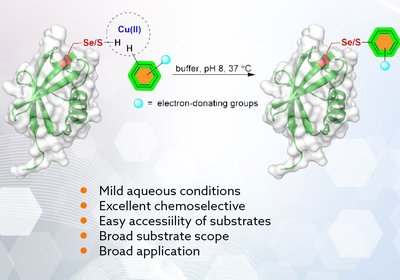
Copper Clicks
Metanis Lab
In a collaborative work between the Metanis and Shimon labs at the Hebrew University of Jerusalem, and the Wang group at the Xiamen University in …
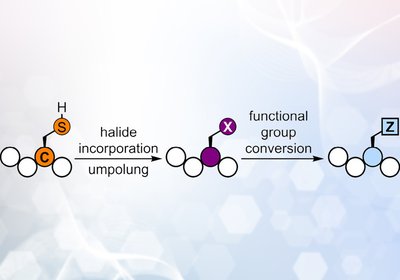
Cysteine Umpolung
Wang Lab
A longstanding challenge in peptide chemistry has been the restricted reactivity of canonical amino acids, which limits late-stage functionalization strategies. Most side chains are inherently …
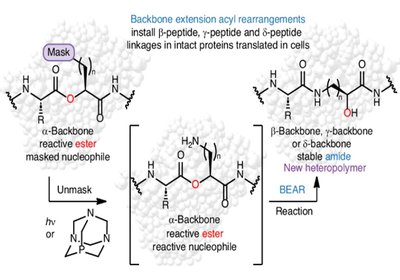
Rewiring the Protein Backbone
Schepartz Lab
Backbone editing offers a powerful way to expand protein function: by introducing β, γ, or δ linkages, researchers can strengthen folds against proteolysis, reshape ligand …
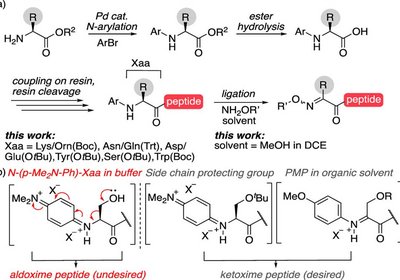
Oxidative Peptide Coupling
Proulx Lab
This study demonstrates that a key determinant of this reactivity is the choice of solvent. By shifting from aqueous buffer to a mixed organic solvent …